Introduction
Cardiac transplantation displays a well established therapeutic procedure for different end-stage heart diseases. Effective immunosuppressive drugs, progress in operative techniques, modern perioperative intensive care, and application of increasingly potent antibiotics in case of postoperative infections led to an improvement in short term outcome of organ transplantation. Nevertheless, achievements in long term transplant results are rare due to rejection of allografts. Chronic graft rejection is the major cause of late transplant failure.
According to present knowledge, T cells, infiltrating monocytes and macrophages, and NK cells, respectively, are involved in acute and chronic rejection. Numerous clinical trials and investigations in animal or cell culture models point out, that cell adhesion molecules, cytokines, and chemokines play a decisive role in the acute and chronic rejection of solid organ grafts.
Heterotopic cardiac transplantation in mice is considered to be the best model to study immunological mechanisms of transplant rejection. This technique allows the analysis of rejection processes in different mouse strains with defined genetic defects. Thus, distinct immunological receptors and ligands can be scrutinized for their impact on physiological and pathophysiological mechanisms of acute and chronic graft rejection. Results achieved from this model could be transferred to human beings in the majority of cases. As such, the model of heterotopic cardiac transplantation in mice has the potential to discover new therapeutic strategies which can be transferred to the clinic.
The aim of this topic is to present our comprehensive microsurgical expertise on this model to other research groups in the field of cardiac transplantation. It delineates the practicable microsurgical model of heterotopic cardiac transplantation in mice as performed in our centre, based on the initially presented technique nearly four decades ago. Furthermore, the necessity of this in vivo model for a detailed understanding of the underlying mechanisms of transplant rejection is going to be discussed.
History and clinical-experimental development of transplantation
Basic requirement for transplantation of vascularized organs was the development of surgical vascular anastomosis techniques, crucially brought forward by Alexis Carrel in Lyon, France at the beginning of the last century [Carrel & Guthrie, 1905]. He successfully performed numerous heart transplantations in dogs and received the Nobel Prize for his research in 1912. In the year 1954 Joseph Murray (Boston, USA) performed the first successful solid organ transplantation in human beings in terms of the first kidney transplantation between monocygotic twins [Merrill et al., 1956]. In 1963 both Starzl (Denver, USA) and Hardy (Mississippi, USA) performed the first liver transplantation and lung transplantation, respectively. The first pancreas transplantation was realized by Richard Lillehei (Minnesota, USA) in 1966 and finally Christian Barnard (Cape Town, South Africa) performed the first human heart transplantation in 1967.
The knowledge about transplant immunology is derived from basic experimental findings during transplantation in animal models. Cellular principles of organ rejection were conclusively described by the English biologist Peter Medawar, who received the Nobel Prize for the "discovery of immunological tolerance" in 1960 [Billingham et al., 1951]. In 1953, histocompatibility antigen was reported for the first time on the surface of leucocytes. Jean Dausset therewith established the basis for histological typing between donor and recipient which is nowadays routinely performed within the pre-operative screening previous to each transplantation, the so-called HLA matching (testing of congruousness of those genes that are responsible for transplant rejection). Nevertheless, T cell mediated immune defence against the graft is not covered in these investigations though T cells take up a key function in transplant rejection [Sayegh & Carpenter, 2004]. This T cell reactivity could be accurately examined in our transplant centre in living donor kidney transplantation between monocygotic twins and in consequence immunosuppression was first reduced and then completely withdrawn [Huser et al., 2009]. To date, medication free development of tolerance or at least reduction of required immunosuppressive drugs remains to be the unchanged goal for increased postoperative transplant survival.
Of course, transplantation of organs between genetically identical individuals continues to be exceptional as transplantation is usually performed in an allogeneic context which implicates the risk of an acute rejection episode. Technical advance and improvements in medicamentous therapy by implementation of potent immunosuppressants lead to an overall one-year graft survival of more than 80% [Christie et al., 2010]. On the other hand, no relevant enhancements in long-term outcome of grafts could be determined. Chronic transplant rejection is hereby the main reason for late graft failure. Besides non-immunological donor- and recipient-dependent factors, mainly immunological reactions represent an important role for graft survival. Heterotopic heart transplantation in mice provides an important and valid model for analysis of immunological events during acute and chronic rejection mechanisms.
Necessity of an in vivo model for investigation of immunological events mediating organ rejection
The reaction against an allograft is composed of a complex cascade of immunological processes and some parts of them can be analyzed in vitro. However, detailed investigation of rejection mechanisms of vascularized grafts includes afferent and efferent steps like e.g. sensitisation of the recipient, antigen processing in lymphoid organs, differentiation and proliferation of immunocompetent cells of the recipient that detect the graft to be "extraneous" and finally direct their movement into the graft. These processes all culminate in graft failure, but they are not reproducible in vitro, because conclusion on the exact genesis and therefore identification of the causality of specific interactions is not possible. Experimental in vivo investigations are especially necessary to display the dynamic of these processes. The animal model is therefore an ideal compromise between clinical reality and experimental reproducibility. The study of genetically modified rodents has become commonplace within immunological research. Sophisticated advances in gene-altered models make it possible to appoint the function of a certain interaction of specific gene products in the context of graft rejection. Within these gene replacement (knock-in) or loss of function mutation (knock-out), mice have been generally accepted for both technical and nontechnical reasons. An observed phenotype can provide clues to the mechanisms of transplant immunology. Moreover, the use of inducible transgenic systems enables to control the location and time of transgene expression in certain tissues and avoids lethal deletion in knockout mice or compensation by various gene products. For evaluation of immunological and especially transplant immunological questions, results derived from the mouse model could directly be transferred to human beings for the most part, albeit the observations must be interpreted carefully due to some differences in both the innate and adaptive arm of the immune system [Mestas & Hughes, 2004]. A large number of important gene products that play an important role in this context were first defined in the mouse. Nearly all interaction molecules like T cell-receptors, cytokine-receptors, accessory molecules, and cell-activation-markers are characterized and a large number of genetically well-characterized transgenic and knock-out strains of the molecules are available. So far, use of homologous recombination to modify genes in embryonic stem cells was only feasible in mice because of the absenteeism of germline-competent embryonic stem cell lines in other species. Therefore, the murine model is more useful for the investigations of transplant rejection, although it requires a higher level of microsurgical skill than the technique in rats. It is only recently that Tong et al. have demonstrated stem cell based gene targeting technology in the rat [Tong et al., 2010]. Therefore the rat model might provide an adequate, powerful tool for the advancement of our understanding in transplant immunology in the future. The most frequently applied transplant model is still the heterotopic mouse heart transplantation. This procedure was described for the first time by Corry in 1973 [Corry et al., 1973] and the method was comparable to the cardiac transplantation in the rat as performed by Abbott et al. in 1964 [Abbott et al., 1964] and Ono et al. in 1969 [Ono & Lindsey, 1969].
A technique utilizing the transplantation of a non vascularized heart was established by Fulmer and coworkers, where neonatal murine cardiac tissue was placed subcutaneously into the pinna of the recipients ear [Fulmer et al., 1963]. Using this technique certain aspects of acute rejection have been studied. Nevertheless, the factors that lead to cardiac allograft vasculopathy all interact within the transplanted vessels at the blood / endothelial interface, making a vascularized cardiac transplant model imperative for the studies of chronic graft rejection [Hasagewa et al., 2007]. Other research groups refer to a simplified and technically easier model of vascularized transplantation in which the graft is anastomosed to the cervical vessels [Chen, 1991; Tomita et al., 1997]. However, according to Doenst et al., first, the positioning of the transplanted heart in an infrarenal position is self-guided and less likely to allow torsion, and second, the carotid artery is smaller in diameter than the ascending aorta of the donor, what makes the aorto-aortic anastomosis easier to perform [Doenst et al., 2001]. Finally the decision of which operative procedure is performed depends on the personal preference.
Anatomical specialities of the heterotopic heart transplantation model
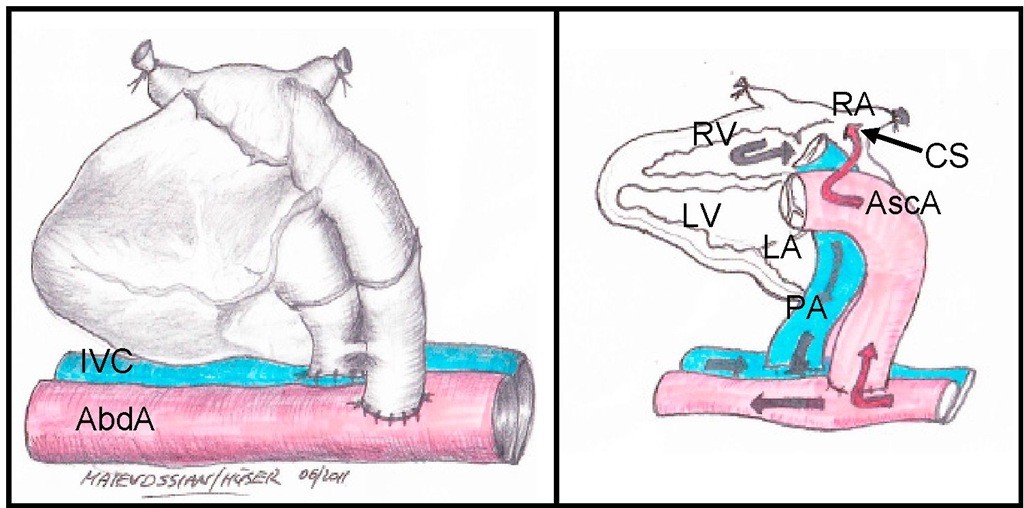
The harvested donor heart is transplanted heterotopically by performing vascular anastomoses of the aorta and the pulmonary artery to the large infrarenal vessels of the recipient [Corry et al., 1973]. Therefore, the recipient mouse is not dependent on the functioning of the graft as its own heart remains untouched and the mouse undergoes rejection of the graft without impairment of physical well-being. A difference of this method compared to orthotopic heart transplantation is related to the technique of vessel anastomoses. The ascending aorta (AscA) of the donor heart is anastomosed end-to-side to the abdominal aorta (AbdA) and the pulmonary artery (PA) is anastomosed end-to-side to the recipient’s inferior vena cava (IVC). This leads to a retrograde blood flow from the abdominal aorta via the ascending aorta directly into the coronary arteries whilst bypassing the left ventricle (LV) and left atrium (LA). After a while a thrombus accrues in the left ventricle. Hence, the oxygen supply of the heart muscle is hereby ensured by the capillary bed. Coming from the coronary arteries, the blood flow then passes the coronary veins and next converges in the coronary sinus (CS) and the right atrium (RA). From the right atrium the blood courses to the right ventricle (RV) and using the stump of the pulmonary trunk it finally reaches the recipients IVC (see fig. 1). The presented model is a so-called „non working heart model", because the graft does neither maintain physiological cardiac output nor pump against physiological pressure.
Fig. 1. Illustration of blood flow in heterotopically transplanted heart cardiac grafts
Options of rejection diagnostics after heterotopic heart transplantation
A relevant advantage of this operative method is the efficient rejection diagnostic of the transplanted heart. An algorithm on investigative technique could be developed to guarantee both the most comfortable examination for the transplanted mouse and the complete and comprehensive acquisition of acute and chronic rejection.
Finger palpation of the transplanted heart is a sensitive method to appraise time-dependent course of rejection. Concerning the contraction power and the induration of the graft, respectively, different stages can be graduated [Schmid et al., 1994]. In case of applying too high pressure, the graft cannot fill up and fully pulsate, and contractility is underestimated [Martins, 2008]. In the hand of an experienced diagnostician, slightest differences, especially the rapidly decreasing contraction power during acute rejection can be measured. In diagnostics of chronic transplant failure, evaluation occasionally might be difficult due to successive impairment of cardial pump functioning, as heart pulsation is directly related to the amount of intact myocard.
The validity of supplementary analysis to get the exact rejection time point remains controversial. Performance of electrocardiogram (ECG) requires exact subcutaneous placement of needle electrodes [Superina et al., 1986], validity is heavily dependent on the organ’s position and movement [Mottram et al., 1988], and therefore the utility of this method is limited. In acute rejection, frequency shows rapid decrease in combination with various ECG alterations [Babuty et al., 1996]. In literature, magnetic resonance imaging is described as an additional tool in diagnostics for assessment of transplanted hearts. Nevertheless, this expensive procedure should be reserved for specific settings only. Moreover, in perfusion studies of solid grafts, results revealed to be extremely dependent on length and depth of narcosis [Wu et al., 2004]. Recent investigations describe high-frequency ultrasound biomicroscopy modality besides conventional echocardiography, a new non-invasive imaging method for diagnostic of acute rejection [Bishya, 2011]. Various papers report on a decrease of end-diastolic diameter and an increase of left ventricular posterior wall thickness, respectively, to be parameters of acute rejection. However, changes in left ventricular posterior wall thickness, seem to be increasingly difficult to measure after the fifth post-operative day, which points to the limitation of the use of echocardiogram in diagnosing acute allograft rejection [Scherrer-Crosbie et al., 2002]. In principle, every final rejection, either acute or chronic, has to be confirmed by diagnostic laparotomy to avoid misjudging passive movement of the graft by transmission of the aortic pulsation as graft beating.
The method of heterotopic heart transplantation in mice
The model of heterotopic heart transplantation in mice has been varied manifoldly since its first description by Robert Corry and Paul S. Russell in 1973 [Mao et al., 2009; Wang et al., 2005; Hasegawa et al., 2007]. The following topic describes the transplantation procedure and incorporates our experience using an operating microscope (OPMI-6, Carl Zeiss, Jena, Germany) and a magnification between 4x-20x objective. Operation time is approximately 45 minutes and peri-operative mortality in the hands of an experienced micro-surgeon is less than 5%.
Anaesthesia and analgesia
All operative procedures in animals are performed using Isoflurane narcosis (Forene with 1-Chloro-2,2,2-trifluoroethyl-difluoromethylether). Both donor and recipient mouse can be operated under pain-free, unconscious, and relaxed conditions. Isoflurane narcosis brings along the crucial advantage of easy handling and having almost no influence on the animal’s blood pressure in contrast to other narcotics such as e.g. Ketanest. Furthermore, Isoflurane narcosis is the least hepatotoxic narcotic drug. Basal anaesthesia is performed with 5% Isoflurane and for maintainance approximately 2% Isoflurane are necessary. Endotracheal anaesthesia is not necessary. Animals reliably wake up approximately 10 min after the end of narcosis.
Donor operation and preparation ex situ
The mouse intended for donor operation receives narcosis in the above mentioned way. Afterwards, the mouse which lies on its back gets fixed with tape at its limbs to a corkboard and Isoflurane is applied via a tube to the mouse’s nose. Hereby, interim awakening of the animal can be avoided.
The operation starts with median laparotomy. The abdomen is kept open by use of two needles, fixing the peritoneum to the corkboard. The bowel gets enveloped into a moist compress and put away to the right side outside the mouse. Then the IVC and the abdominal aorta can be prepared with a cotton swab. Using 1 ml syringe and 25G needle, the Aorta is canulated and as much blood as possible is aspirated. The initially performed abdominal cut to the lower margin of the left hepatic lobe has to be extended to the sternum. Next, two more cuts have to be done along the costal arches to enable separation of the diaphragm from its costal and sternal adherence. In the following step the thorax is opened by cutting along the midaxillary line right up to the upper thorax aperture. Fixation of the sternum above the mouse’s head (e.g. with a needle holder) guarantees free access to the opened thoracic cavity. Via puncture of the IVC the heart can now be flushed with cardioplegic fluid and it stops beating. After preparation and resection of the thymus gland, the inferior vena cava and the superior vena cava (SVC) are ligated with 6/0 silk. Silk ties are placed around the right and left pulmonary vessels (RPV / LPV) to exclude the lungs. Finally, the azygos vein (AV) is ligated. The donor heart is gently detached from the surrounding tissue with blunt dissection. For anastomosis of the recipient’s abdominal aorta (AbdA) the donor’s Aorta ascendens (AscA) and pulmonary artery (PA) remain open (see fig. 2). By now the heart can be harvested by sharply dividing along the esophagus and cutting the ligated vessels. The ascending aorta is cut below the brachiocephalic artery and the main pulmonary artery is cut proximal to its bifurcation.
Preparation of the pulmonary trunk and the Pars ascendens aortae has to be performed ex situ. Both of them are piggybacked on a curved pair of pincers and cut with a micro-surgical pair of scissors. Hereby you get two homogeneous and relatively long vessel stumps that enable easy implantation during the recipient operation. Until this moment the cardiac graft has to be stored in 4°C cold cardioplegic solution (e.g. Bretschneider’s cardioplegic solution, Kohler Chemie, Alsbach, Germany).