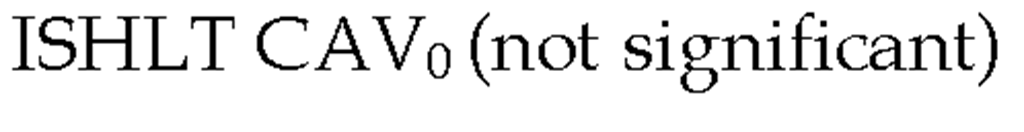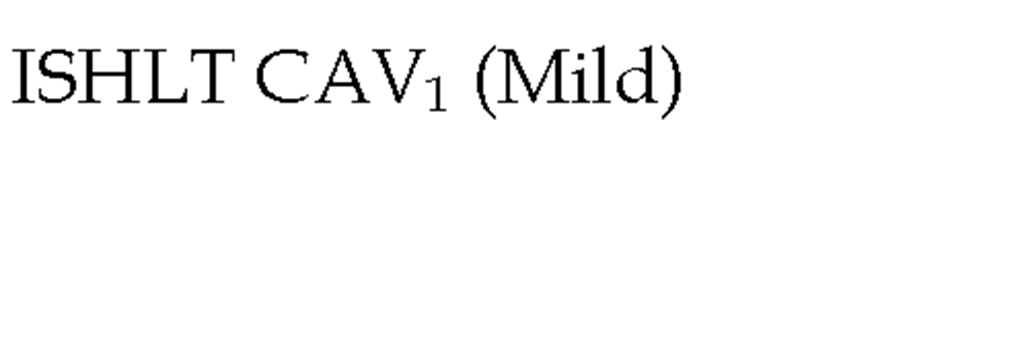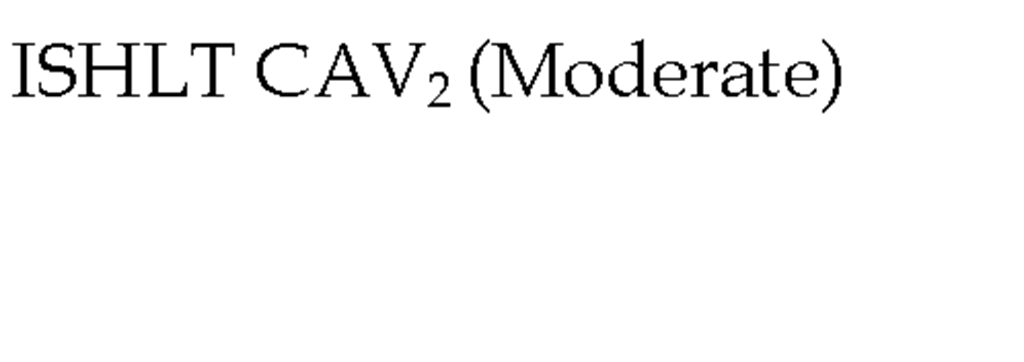Donor-recipient factors
Donor-recipient factors that increase risk of CAV include episodes of high-grade cellular rejection during the first year after transplantation, HLA-DR mismatches, antibody mediated rejection (including asymptomatic AMR), and cytomegalovirus (CMV) infection.
The total cellular rejection score at six months increases the risk of CAV by almost threefold (Raichlin et al., 2009). The total cellular rejection score is assigned based on ISHLT grading as 0R = 0, 1R = 1, 2R = 2, 3R = 3, and normalized by dividing the cumulative scores for the total number of biopsies taken during the 6 month intervals. Antibody-mediated rejection increases the incidence of CAV by 10% at one year and by 36% at five years (Michaels et al., 2003). Asymptomatic AMR, defined as histologic evidence of AMR, including endothelial swelling, interstitial hemorrhage, interstitial edema and neutrophil infiltration and/or immunoperoxidase staining showing CD-68 positive macrophages within capillary cells and C4d complement coating the walls of the myocardial capillaries, in patients without symptoms or any evidence of graft dysfunction, increases the risk of developing CAV compared to those with no AMR (We et al., 2009).
CMV disease appears to increase the risk of early-onset CAV -an effect that may be mediated via immune mechanisms rather than a direct consequence of the infective organism (Weill et al., 2001). CMV infection in endothelial cells, such as those lining the donor coronary arteries, leads to a local proinflammatory state and subsequent increased production of vascular cell adhesion molecule (VCAM)-1, alterations in cell surface MHC I and II molecules, and an increase in proinflammatory cytokines and growth factors. This causes endothelial cell/ smooth muscle cell proliferation and narrowing of the vessel lumen. CMV may also cause endothelial dysfunction leading to CAV by impairing the nitric oxide synthase pathway. (Hosenpud et al., 2003; Koskinen 1993; Van Dorp et al., 1989). Nitric oxide prevents vascular inflammation and lesion formation in the vessel wall by inhibiting platelet and leukocyte adherence and by suppressing vascular smooth muscle cell proliferation. CMV infection of the endothelium increases expression of endothelial surface adhesion molecules and promotes mononuclear adhesion, activation, and transendothelial migration within the vasculature. In addition, CMV is a stimulus of plasma ADMA (asymmetric dimethylarginine) accumulation, the endogenous inhibitor of nitric oxide synthase. (Weis M et al., 2004)
|
Donor Factors |
|
Age (>50 years), Sex (male) and Mode of Death |
|
History of hypertension, diabetes, smoking ? Donor Transmitted Atherosclerosis |
|
Hepatitis B & C infection |
|
Recipient Factors |
|
Age (>40 years), Sex (male) |
|
Hypertension, dyslipidemia, obesity, smoking |
|
Insulin resistance/Diabetes |
|
Hepatitis B |
|
Donor – Recipient Interaction |
|
Rejection (cellular and antibody mediated) |
|
CMV disease |
Table 2. Risk factors for the development of cardiac allograft vasculopathy
Clinical manifestations
Early CAV is clinically silent, and ischemia is usually not evident until the disease is far advanced. Patients are most often diagnosed by routine angiographic surveillance or loss of allograft function by surveillance imaging with negative rejection studies. Clinically, patients present with heart failure symptoms and are found to either have new onset systolic or diastolic dysfunction in the absence of acute cellular or antibody mediated rejection, or may present with ventricular arrhythmias and sudden cardiac death. Patients rarely present with chest pain, due to de-innervation of the cardiac allograft at the time of transplantation. However, 25-40% of patients develop some degree of re-innervation post transplant, manifest primarily by return of heart rate response and less often, ischemic chest pain (De Marco et al., 1995).
Epidemiology and natural history
CAV is detectable by coronary angiography in approximately 10% of heart transplant recipients within the first year, in 32% – 50% by 5 years, and in an even greater number of recipients when detected by intravascular ultrasound (Pflugfelder et al., 1993). Once mild disease is identified angiographically, the likelihood of progression to severe CAV within 5 years is approximately 19% (Costanzo et al., 1998). Lack of development of CAV at 1 year post-transplant identifies a group who are likely to remain free of clinical events through 6 years of follow up (Luyt et al., 2003). In patients with at least 1 focal stenosis >40%, survival was 67% at 1 year, 44% at 2 years, and 17% at 5 years. With 3 vessels involved, survival was 13% at 2 years (Keogl et al., 1992). The overall likelihood of death or re-transplantation as a result of CAV is approximately 50% for severe CAV (Costanzo et al. 1998).
Graft function is an important component in evaluating severity of CAV. Patients with CAV and LV dysfunction have significantly lower 5-year survival compared with CAV patients without LV dysfunction (60% vs. 90%) (Stork et al., 2006). Patients with restrictive cardiac physiology [E to A velocity ratio >2, shortened isovolumetric relaxation time (<60msec), shortened deceleration time (<150ms), or restrictive hemodynamics (right atrial pressure >12mmHg, pulmonary capillary wedge pressure >25mmHg, cardiac index <2.0)] in the presence of preserved LV systolic function and CAV also have a lower 5-year survival than heart transplant patients without restrictive physiology (Itagaki et al., 2007).
Diagnosis
Coronary angiography and assessment of cardiac allograft function remains the cornerstone for diagnosis of CAV. Anatomic abnormalities characteristic of CAV include gradual, distal, diffuse, concentric tapering ("distal pruning") of the major epicardial arteries with obliteration of the small branching vessels. Discrete epicardial stenoses are less likely (Figure 3). As early CAV is asymptomatic, routine screening with annual coronary angiography (with or without intravascular ultrasound) is standard practice among the majority of transplant centers in the first 3-5 years post transplant. After this time period, there are different practice patterns for assessment of CAV, depending on individuals’ risk factor profile for CAV, graft function, renal function, and clinical status. As CAV is a concentric process, early disease may be overlooked if prior angiograms are not compared. For this reason, baseline angiography is usually performed 4-6 weeks post transplant.
In 2010, the ISHLT working group proposed standardized nomenclature for CAV (Table 3) (Mehra et al., 2010). This system defined CAV as not significant, mild, moderate, and severe based on angiographic appearance. Angiography is a very useful test for detecting lesions located in epicardial arteries and the main branches, however the main limitation of angiography is low sensitivity, especially in those mild cases in which an apparently normal examination can underestimate the presence of CAV. This is primarily due the vascular remodeling that occurs early in the disease in which there is no compromise in luminal diameter (Nissen 2001). Repeated angiography is recommended for the follow-up of patients with moderate lesions or for patients with symptoms leading to the suspicion of CAV.
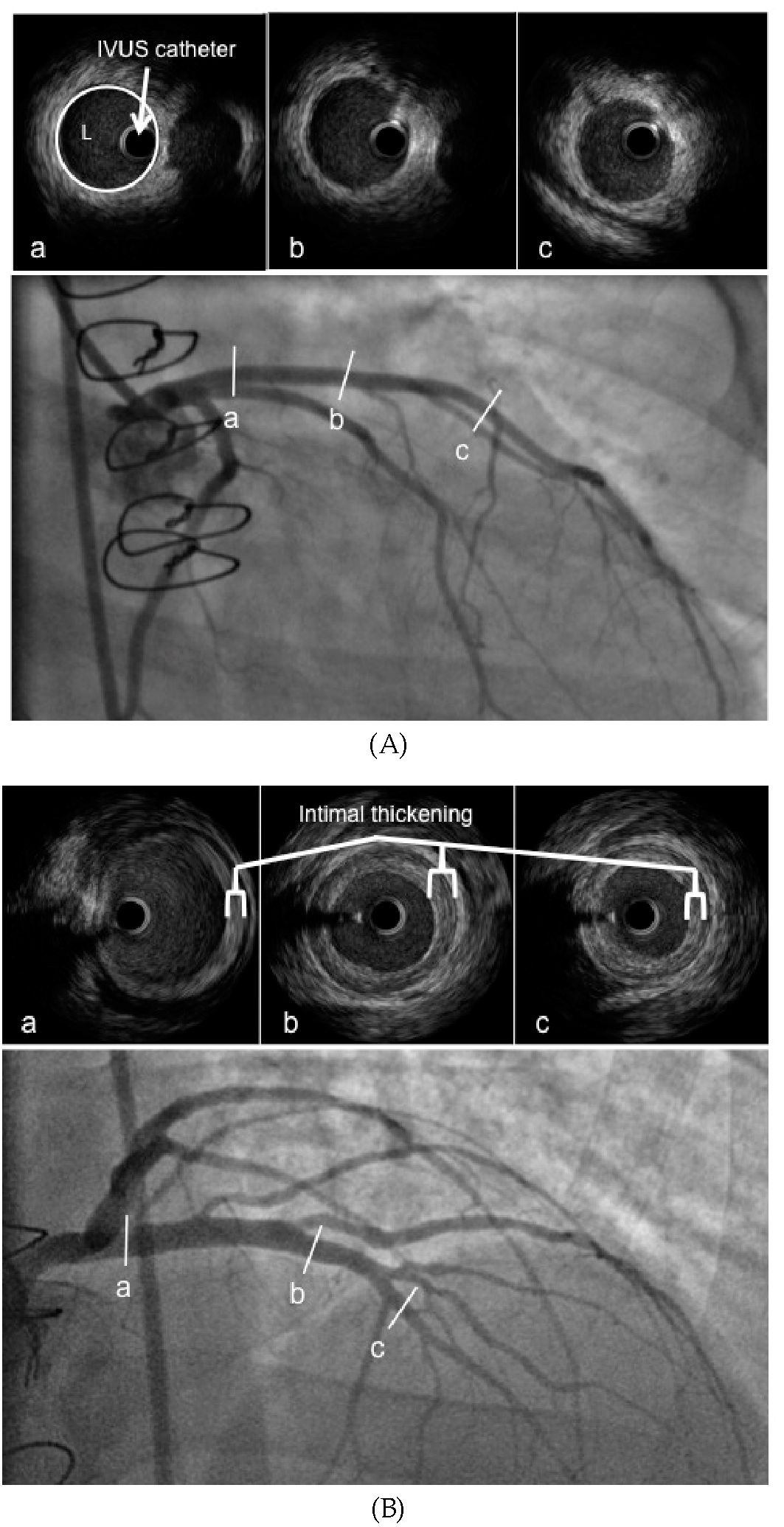
Fig. 3. Angiography and IVUS imaging demonstrating (A) normal coronary arteries and (B) cardiac allograft vasculopathy. (L= coronary artery lumen).
Intra-vascular ultrasound (IVUS) at coronary angiography has been evaluated as an adjunctive modality for assessing and diagnosing early CAV. Angiography depicts only a 2-dimensional silhouette of the lumen whereas IVUS allows a reproducible view of both actual lumen diameter, as well as the appearance and thickness of the intima and media. As the intima begins to thicken, the vessel area enlarges as a compensatory mechanism to preserve luminal area. Therefore, ‘angiographically normal’ coronary arteries may in fact have significant CAV.
IVUS parameters include intimal thickness, intimal index, and change in maximal intimal thickness at a reference point, total atheroma volume, and percentage of atheroma volume. IVUS can detect occult disease in angiographically normal sites and has emerged as the optimal diagnostic tool for early detection. CAV is considered present when the intimal thickness is >0.3mm (Rickenbacher et al., 1995). The presence of moderate to severe intimal thickening by IVUS is predictive of the future development of angiographically apparent CAV. IVUS-detected maximal intimal thickness (MIT) greater than 0.3mm at 1 year is associated with a 4-year actuarial survival of 73% versus 96% among those with less than 0.3mm of intimal thickening (Rickenbacher et al., 1995). A change in MIT of > 0.5mm at a specific site in the coronary tree within the first year after transplant predicts outcomes at 5 years related to the development of angiographic CAV, mortality and myocardial infarction (Kobashigawa et al., 2005; Tuzcu et al., 2005). Death or graft loss occurred in 21% versus 6%, non-fatal major adverse events or death/ graft loss in 46% versus 17% and angiographically apparent disease in 65% versus 33% in those with a change in MIT > 0.5mm between baseline and 1-year (Kobashigawa et al., 2005) versus those with a lesser degree of intimal thickening. With >0.6mm intimal thickening, patients are 10 times more likely to experience a cardiac event and for those who developed cardiac events, 62% of patients had ‘normal’ angiograms (Mehra et al., 1995).
In patients with stable graft function and no history of CAV, most centers alternate coronary angiography with dobutamine stress echocardiography after 3-5 years; reserving invasive catheterization to every other year. The sensitivity and specificity of dobutamine stress echocardiography compared with coronary angiography and IVUS for detecting CAV is approximately 80% and 88% respectively (Derumeaux et al., 1995; Spes et al., 1999). A normal DSE incorporating M-mode measurement of wall thickening predicts an uneventful clinical course (Spes et al., 1999).
Computed tomographic angiography, although useful in detecting traditional atherosclerosis, has limitations in heart transplant recipients. The major drawbacks with the routine use of 16 or 64 slice multidetector CT in this population include the high heart rate of most heart transplant recipients (due to cardiac vagal denervation) that may compromise imaging quality, contrast-induced nephropathy and radiation. However, compared with IVUS, the sensitivity, specificity, positive and negative predictive values for the detection of CAV by dual source CT were 85%, 84%, 76%, and 91% respectively (Schepis et al., 2009). Magnetic resonance coronary angiography, at present, shows limited sensitivity for detecting CAV and positron emission tomography (PET) has not yet been well studied (Muehling et al., 2003).
Table 3. The International Society for Heart and Lung Transplantation Recommended Nomenclature for Cardiac Allograft Vasculopathy. ‘Primary vessel’ denotes the proximal and middle 33% of the LAD, circumflex, ramus and the dominant or co-dominant right coronary artery with the posterior descending and posterolateral branches. ‘Secondary branch vessel’ includes the distal 33% of the primary vessels or any segment within a large septal perforator, diagonals and OM branches or any portion of a non-dominant RCA. Restrictive physiology is defined as symptomatic heart failure with echocardiographic E to A velocity ratio >2, shortened isovolumetric relaxation time (<60msec), shortened deceleration time (<150msec) or restrictive invasive hemodynamics (right atrial pressure >12mmHg, pulmonary capillary wedge pressure >25mmHg, cardiac index <2L/min/m2)
Serum biomarkers provide important clues to molecular and structural changes within the transplanted heart. The role of inflammation has been demonstrated by the elaboration of inflammatory cytokines in patients with CAV. Elevated CRP levels have been shown to be associated with increased risk of developing CAV, suggesting a link between systemic and local inflammation within the coronary artery of the transplanted heart (Arora et al., 2010; Raichlin et al., 2007). However, it is not clear if CRP is merely a marker or if it is involved in CAV pathogenesis. Persistently elevated troponin I levels after heart transplantation are associated with an increased risk of development of CAV (Labarrere et al., 2000). Elevated BNP has also been correlated with the development of CAV (Mehra et al., 2004b). BNP may be useful in combination with angiographic findings in predicting outcomes, with 50% of patients with high BNP levels and angiographic CAV experiencing cardiac death (Tsutsui et al., 2001). Biomarkers may hold predictive correlation, but are not used routinely for the evaluation of CAV.
Prevention and treatment
Therapeutic options in CAV can be thought of in terms of prevention and treatment. Medical therapy aims to slow, halt, or even reverse the intimal proliferation, concentric luminal loss and arterial obliteration that characterize CAV. Preventive therapy targets traditional cardiac risk factors (diabetes mellitus, hyperlipidemia and hypertension) and aggressive treatment of rejection episodes. Rapidly progressive CAV within the first year after heart transplant strongly predicts all-cause mortality, myocardial infarction, and the subsequent development of angiographically severe CAV. Accordingly, prophylactic strategies must be implicated very early to induce significant improvement in long-term prognosis.
Statins have been associated with reduced progression of CAV, and routine administration of statins to all patients after transplant has clearly reduced the mortality and progression of the disease (Wenke et al., 2003). A meta-analysis based on the published results of three randomized clinical trials determined that the use of statins decreased 1-year mortality following heart transplantation from 17.1% to 5.4% (Mehra et al., 2004c). The introduction of routine anti-CMV drugs to all CMV seropositive patients (donor or recipient or both) for at least 3 months and in some centers up to 6 months has lowered the incidence of CMV infection and its impact on the graft. In a randomized study, patients receiving gancyclovir were significantly more likely to remain free of CAV for 5 years post transplant (Valantine et al, 1999).
There are small studies that suggest calcium channel blockers, such as diltiazem, are effective in slowing the progression of CAV (Schroeder et al., 1993). ACE inhibitors have been associated with plaque regression and improved survival for established CAV , however pre-emptive therapy with ACE inhibitors has unknown preventative effects (Bae et al., 2006). Currently, there is little definitive evidence to suggest that calcium channel blockers or ACE inhibitors are effective in reducing the incidence or slowing the progression of CAV.
Diabetes is common in cardiac transplant recipients and most heart transplant patients are markedly insulin resistant and demonstrate many of the features of compensatory hyperinsulinemia, including the atherogenic and proinflammatory profile of the metabolic syndrome (Potena L & Valantine H, 2007). Patients with high plasma glucose or insulin concentrations after oral glucose intake have greater intimal thickness and are less likely to be free of CAV and have significantly lower survival during 5 years of follow up than patients with low glucose and insulin levels (Valantine et al., 2001). Specific therapies shown to be effective in correcting insulin resistance, such as the thiazolidinediones, offer potential new therapeutic targets for CAV prevention, however the clinical studies are lacking.
Owing to the diffuse nature of vasculopathy, conventional therapeutic approaches including percutaneous coronary intervention and coronary artery bypass grafting are optional only late in the course and are not always successful or even feasible.
Immunosuppression continues to represent the principal means of pharmacologic prophylaxis against and treatment of allograft vasculopathy, with the development and introduction of the proliferation signal inhibitors (PSI) sirolimus and everolimus representing the greatest advance achieved to-date in this field. PSI’s are powerful immunosuppressant drugs that inhibit cellular proliferation and have shown the ability to reduce, and even reverse, coronary intimal growth in established CAV and reduce the incidence of CAV in de novo transplant patients through a variety of mechanisms, including a reduction in CMV infection and decreased rejection episodes. (Eisen et al., 2003; Keogh et al, 2004).
In a prospective multi-center randomized double blind control trial, patients who received everolimus, cyclosporine and steroids versus azathioprine, cyclosporine and steroids had a significant reduction in the incidence of CAV at 12 months (35.7% versus 52.8%), fewer grade > 3A cellular rejections (21.3% versus 30.6%) and CMV infection (12% versus 41.4%) (Eisen et al., 2003). Similar results were found with sirolimus (Keogh et al., 2004). The long-term results of both studies demonstrate a trend toward a lower incidence of severe vasculopathy in the group that is maintained on PSI with patients treated with everolimus having significantly lower rates of major cardiovascular events related to CAV compared to patients treated with azathioprine (7.9% vs. 13.6%) (Eisen et al., 2006; Keogh et al., 2007).
The available data suggest the efficacy of PSIs in reducing the incidence of CAV; however, up to 30-40% of patients in the clinical trials had to discontinue the PSI during the first year because of poor tolerability. Complications and side effects include bacterial and viral infections (17-30%), oral ulcerations, pericardial effusions (up to 25%) and pleural effusions (15-40%), hematologic abnormalities including anemia and thrombocytopenia, impaired wound healing, acne, and pedal edema. Adverse events may be related to the dosing regimen (high vs. low dose) and timing (immediately post transplant vs. delayed introduction). A recent study with lower serum concentrations of everolimus (3-8ng/ mL) report a lower drop-out rate of 15% (Lehmukuhl et al., 2009). At this time, it remains unclear whether patients should be transitioned to a PSI-based immunosuppressant regimen once sternal healing has occurred.
In relation to the pharmacologic treatment of established CAV, aggressive control of traditional cardiac risk factors is important; however, the PSIs represent the most important advance. Studies in which patients with established CAV were switched to a PSI from MMF or azathioprine demonstrated a significant reduction in CAV-related adverse events (5% vs. 25%) including death, percutaneous coronary intervention (PCI), myocardial infarction, or angiographic progression of the disease 2 years following the transition and a reduction in plaque size compared to progression (Mancini et al., 2003; Segovia et al., 2004). Larger studies are needed to corroborate these initial findings; however, it is generally accepted practice that patients with established CAV are transitioned to PSI.
The treatment of established CAV represents a clinical challenge. Non-pharmacologic treatment includes PCI, coronary artery bypass grafting (CABG), and retransplantation. Percutaneous and surgical revascularization is limited by the diffuse coronary involvement, the infrequency of focal lesions with suitable distal targets, and the high mortality rates with surgical intervention (up to 30-40%) (Musci et al., 1998; Patel et al., 1997). PCI is associated with excellent short-term results, however is associated with high restenosis rates. With the advent of drug eluting stents, preliminary studies suggest much lower restenosis rates. (Lee et al., 2008). PCI remains a palliative therapy, as CAV is a diffuse and progressive disease process. It may be appropriate to perform PCI in patients with discrete focal lesions with abnormal graft function or evidence of stress-induced functional significance by stress imaging. Retransplantation is the only definitive therapy for CAV; however, survival is lower than after primary heart transplant and the probability of CAV in the retransplanted heart is higher than in de novo transplants (up to 50% at 3 years) (Musci et al., 1998; Srivastava et al., 2000). Re-transplantation is reserved for highly selected patients with CAV.
Summary
The development of CAV has been described as the Achilles heel of cardiac transplantation. The increased mortality observed in the years after the diagnosis of CAV reflects that fact that, at present, the management of these patients represents a clinical challenge. Diagnosis is heavily reliant on invasive testing and early diagnosis and preventive strategies are crucial, but remain ineffective in many cases. Statins, antihypertensive drugs, and anti-CMV agents have all demonstrated a modest beneficial reduction in CAV; however, the proliferation signal inhibitors represent the best prevention and treatment options available at this time. Current research focuses on identification of novel agents that are effective in preventing the development and progression of CAV, and non-invasive techniques for CAV diagnosis.