Introduction
Cardiac allograft vasculopathy (CAV) represents the most common cause of late graft failure and limits the long-term success of heart transplantation. It is the most common cause of death, in addition to malignancy, in patients who survive the first year after transplant (Taylor et al., 2006). CAV is a diffuse, accelerated form of coronary artery disease in the transplanted heart. Despite improvements in immunotherapy, the incidence of angiographically detected CAV has not changed appreciably over the past two decades. CAV is associated with a relentless course after heart transplantation and immunological and non-immunological interventions have only partially modified its natural history. Treatment of CAV is limited, highlighting the importance of preventive therapies. Re-transplantation for advanced disease is the only definitive therapy, but is limited to select patients. In this topic, we will review the epidemiology, natural history, clinical manifestations, screening and diagnostic strategies for CAV while emphasizing current treatment strategies designed to prevent or slow the progression of this disease.
Pathology
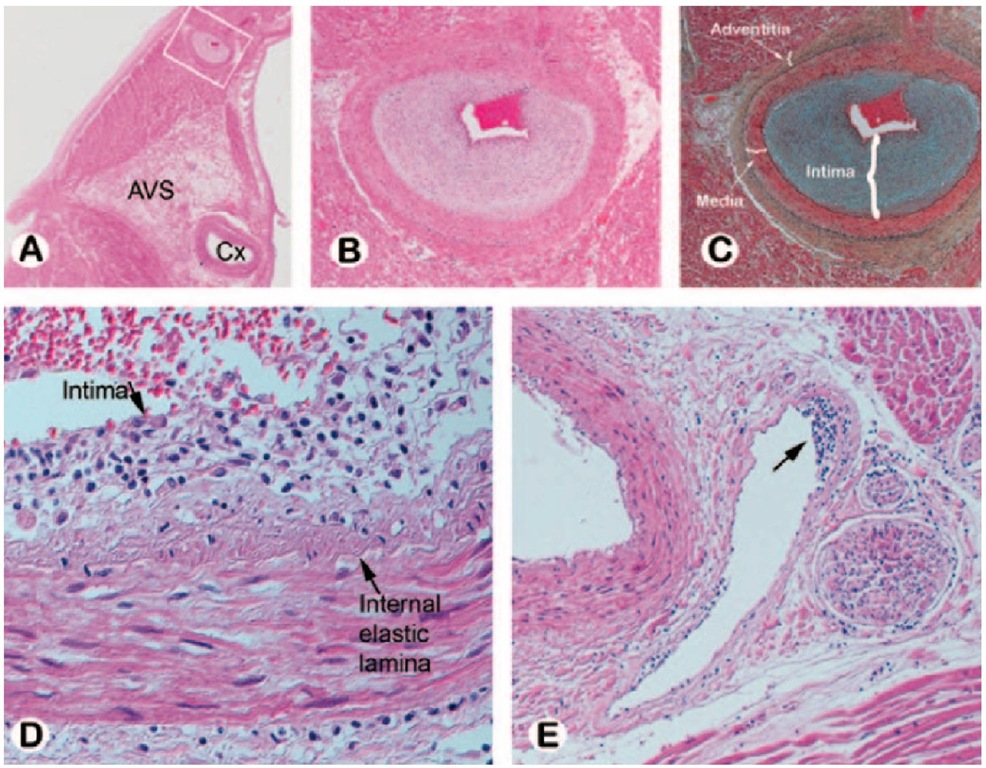
CAV was first described in 1970 as a diffuse, obliterative, accelerated form of arteriosclerosis (Bieber et al., 1970). Pathologically, CAV is distinctly different from traditional coronary atherosclerosis (Table 1). It is characterized by diffuse, concentric intimal smooth muscle hyperplasia involving the entire circumference and length of the vessel, and deposition of neointima in the major epicardial vessels and microvasculature, which progressively obstructs the affected vessel lumen (Figure 1). CAV is further distinguished microscopically as having intense cellular proliferation, composed mainly of smooth muscle cells and inflammatory infiltrates (monocytes and lymphocytes). Specific populations of lymphocytes co-localized with CAV lesions include recipient CD4+ T cells, thus suggesting a role for major histocompatibility complex (MHC) II antigen recognition and processing in the development of CAV. Populations of macrophages and CD8+ T cells have also been localized in CAV lesions, whereas recipient B-cells are rare.
The endomyocardial biopsy has limited sensitivity in the recognition of vasculopathy because it samples only the smallest of intramyocardial arteries and arterioles, which often do not show histologic features of CAV. Reported histologic changes seen in the small arteries in endomyocardial biopsies include concentric intimal thickening with or without foamy macrophages, subendothelial accumulation of lymphocytes, and perivascular fibrosis (Pardo et al., 1997). Evidence of myocardial ischemia, such as myocytolysis, frank coagulation necrosis, and healing ischemic lesions as well as interstitial, perivascular, and replacement fibrosis can be seen (Neish et al., 1992). Identification of myocardial injury should raise the suspicion of CAV as the cause of graft dysfunction. The absence of these findings, however, does not necessarily rule out the presence of CAV.
Pathogenesis
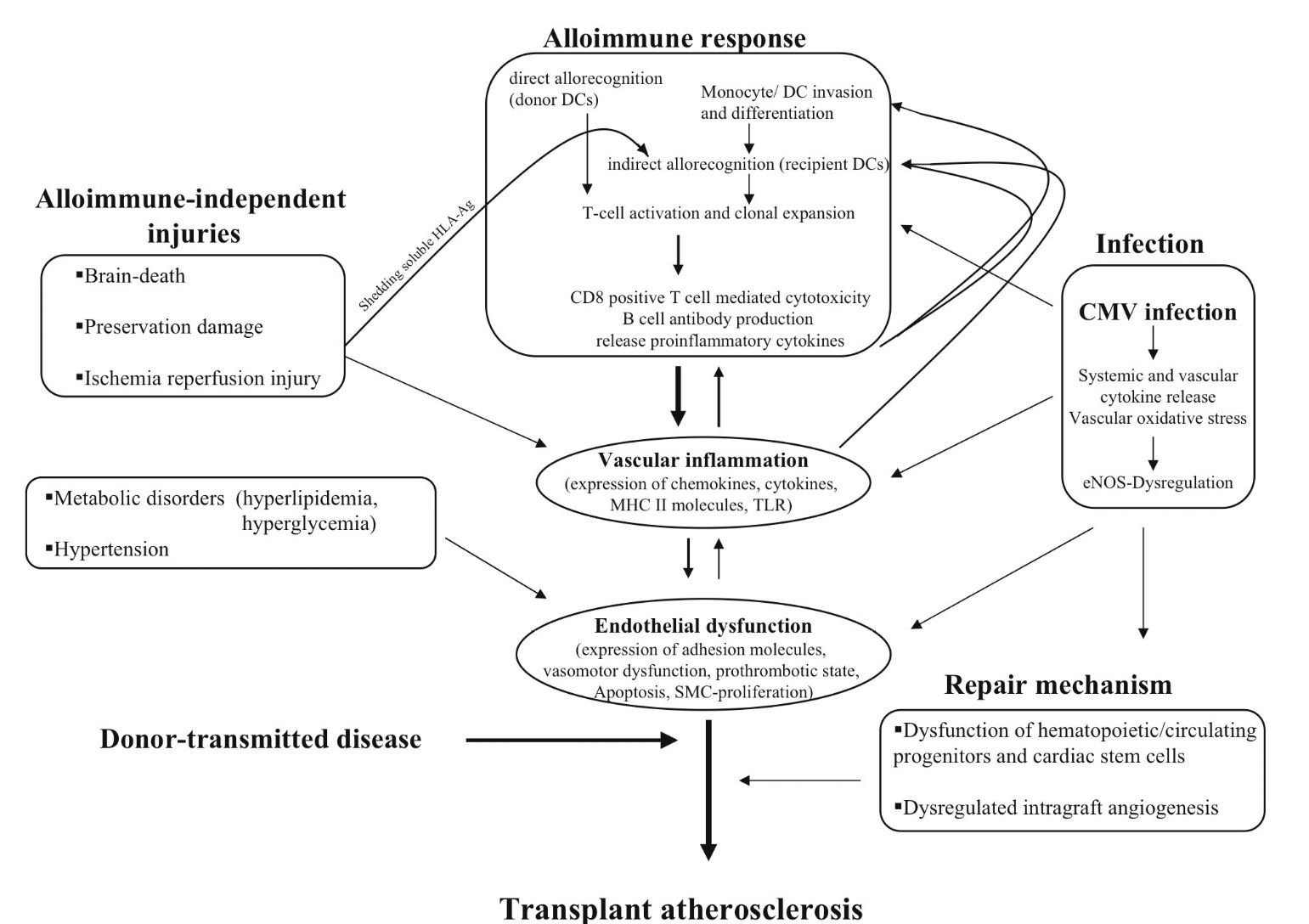
The development of CAV represents a complex interaction between acute and chronic immune activation via an allo-immune pathway and alloantigen-independent non-immune mechanisms that ultimately lead to inflammation and endothelial injury, the precursor to CAV. (Figure 2). Immunologic events appear to be most important, since CAV develops in the donor’s coronary arteries but not the recipient’s vasculature. The donor coronary artery endothelial cells express major histocompatibility complex (MHC) class I and class II antigens, and thus appear to be primary targets of the cell-mediated and humoral immune responses. These antigens are thought to be recognized by recipient CD8+ and CD4+ T cells and these activated lymphocytes secrete cytokines (interleukins, interferons, and tumor necrosis factors), which promote proliferation and further activation of allo-reactive T cells, monocytes, and macrophages. Macrophages are then recruited to the intima where they elaborate cytokines (IL-1, IL-6, tumor necrosis factor TNF alpha) and growth factors [platelet derived growth factor (PDGF), insulin-like growth factor-I (IGF-I), transforming growth factor-alpha (TGF-alpha), TGF-beta-1], leading to smooth muscle cell proliferation and synthesis of extracellular matrix (Libby P & Tanaka H, 1994).
T cell activation also stimulates the expression of adhesion molecules, [intercellular cell adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), P-selectin] thereby "activating" endothelial cells, enabling recruitment of proinflammatory cells from the vasculature and the initiation of the immune response and subsequent development of CAV.
|
Pathologic characteristic |
CAV |
Native CAD |
|
Lesion morphology |
Diffuse, concentric (variable) |
Focal, proximal, eccentric |
|
Vessels involved |
Intramyocardial, epicardial branches |
Epicardial, spares intramyocardial branches |
|
Collateral vessels |
Minimal |
Often extensive |
|
Initial Lesion |
Smooth muscle proliferation |
Fatty streaks |
|
Internal Elastic Lamina |
Intact |
Disrupted |
|
Endothelial lymphocytes |
Sometimes |
Absent |
|
T-cell location |
Subendothelial |
Edge of plaque |
Table 1. Characteristics of cardiac allograft vasculopathy compared to traditional coronary atherosclerosis in the native heart.
Fig. 1. Cardiac allograft vasculopathy. (A) Photomicrograph of the left atrium and ventricle with the left circumflex (Cx) artery noted at the atrioventricular sulcus (AVS) from a patient who died of CAV 3 years post transplant. (B,C) Intramyocardial branch of circumflex artery (from inset A) supplying the atrium shows marked intimal proliferation with preservation of the elastic lamina and normal medial layer (hematoxylin-eosin (B) and Movat pentachrome (C)). (D,E) Subendothelial lymphocytic infiltration is seen in a small epicardial artery (D) and in an intramyocardial vein (arrow E). Reprinted from Tan CD, Baldwin WM, Rodriguez ER. Update on Cardiac Transplantation Pathology.
Non -immune mechanisms implicated in the development of CAV include inflammatory injury due to recipient hypertension, dyslipidemia, diabetes and insulin resistance, and obesity, in addition to ischemia-reperfusion injury during organ procurement. Endothelial cell dysfunction resulting from inflammatory injury predisposes to thrombosis, vasoconstriction, and vascular smooth muscle cell proliferation. Reperfusion injury increases expression of endothelin 1, which is associated with post-transplant ischemic fibrosis and subsequent development of arteriopathy, in addition to generating free radicals that promote the expression of such inflammatory adhesion molecules and subsequent leukocyte recruitment (Ferri et al., 2002). More research is needed in the field of organ preservation, possibly targeting specific cellular processes to minimize reperfusion injury. Endothelial injury and dysfunction, regardless of the mechanism, seems to be the key inciting event and the common pathway for the development of CAV.
Risk factors
Many risk factors for the development of CAV have been identified and can be broadly divided into donor-specific factors, recipient-specific factors and donor-recipient factors, with the latter being predominantly immune mediated (Table 1).
Donor factors
Donor factors include a history of hypertension, diabetes, male sex, and history of tobacco use. Older donor hearts confer a higher risk of CAV development compared to younger donor hearts, and this risk appears most significant with donors older than 50 years (Al-Khaldi et al., 2006; Nagji et al., 2010).
Donor mode of death has also been shown to have associations with the development of CAV. Explosive modes of brain death, including gunshot wound to the head, accidental head trauma, or a rapidly progressive intracranial hemorrhage have also been associated with development of CAV after transplantation (Mehra et al., 2004a). It has been demonstrated that rapid brain death induces a calcium overflow injury that affects conduction tissue, coronary artery smooth muscle, and myocardial cells (Cohen et al., 2007; Novitzky et al., 1988). There is also a generalized activation of macrophage-associated cytokines, an up-regulation of immunoregulatory and adhesion cell molecules with subsequent endothelial inflammation and dysfunction, in addition to increased levels of circulating catecholamines, all of which may negatively impact the graft response after heart transplantation (Segel et al., 2002; Takada et al., 1998).
The role of transmitted native vessel atherosclerosis is controversial and conflicting. Studies utilizing serial coronary artery intravascular ultrasound (IVUS) found donor lesions did not significantly change after transplantation (Jimenez et al., 2001). However a maximal intimal thickness > 0.5mm at baseline (1 month post transplant) was one of the strongest predictors of plaque progression at one year in another study (Yamasaki et al., 2011). In another observational study, donor lesions (defined by intimal thickness > 0.5mm by IVUS at 1 month post transplant) did not have a greater increase in intimal thickness compared to other sites without donor lesions, however the incidence of angiographically apparent CAV up to three years after transplant was higher in patients with donor transmitted lesions with no apparent effect on 3 year mortality (Li H et al., 2006).
Hepatitis C infection (HCV) in donor hearts increases recipient mortality with mortality rates in one study of 16.9% at 1 year, 41.8% at 5 years, and 50.6% at 10 years, compared to recipients of hepatitis C negative donors who had 8.2%, 18.5%, and 24.3% mortality, respectively (Gasink et al., 2006). Recipients with HCV-positive donor hearts were more likely to die secondary to CAV. Other studies also note higher rates of CAV in recipients of HCV + donor hearts, possibly related to HCV viremia in a significant proportion of these recipients; the exact mechanism however, is not completely understood (Haji et al., 2004). Hepatitis B seropositivity (HBV) has been correlated with increased rates of CAV. In one observational study, HBV seropositivity, defined as either the donor or recipient being hepatitis B core antibody positive, was associated with higher rates of CAV at 1 year compared to cases where neither the donor nor recipient were HBV seropositive (Haji SA et al., 2006).
Recipient factors
Recipient factors that are associated with the development of CAV include male sex, pre-transplant ischemic heart disease, and higher body mass index (Costanzo et al., 1998; Taylor et al., 2007). Traditional cardiac risk factors in the recipient including hypertension, diabetes, dyslipidemia and smoking also increase the risk of developing CAV (Sanchez et al., 2008). Importantly, hypertension, dyslipidemia and diabetes are commonly induced post transplant as side effects of immunosuppressant therapy with calcineurin inhibitors and steroids. The prevalence of hypertension increases after transplant from 74.4% at one year to 98.5% at 10 years (Taylor et al., 2007). The incidence of diabetes after heart transplant ranges from 15% to 20% (Martinez-Dolz et al., 2005).
Fig. 2. Pathobiology of Cardiac Allograft Vasculopathy.