Regulation
In addition to intrinsic channel defects, there are many regulatory proteins that interact with channels to modulate their activity. Since LQT1 and LQT2 patients often have arrhythmias precipitated by physical or emotional stress, it is important to consider the human stress response affect these channels. The a- and P-adrenergic systems are activated during stress. The P-adrenergic system involves the P-adrenergic receptor, a hetero-trimeric G-protein, and cyclic adenosine monophosphate (cAMP), a second messenger that ultimately activates protein kinase A (PKA). HERG current is acutely reduced by PKA signaling due to direct phosphorylation of the channel. Furthermore, cAMP can interact with the HERG channel directly in a manner that partially abrogates the suppressive effects of phosphorylation. An added complexity to this signaling pathway is the interaction between 14-3-3, a scaffolding protein, and HERG (Kagan et al., 2002; Kagan & McDonald, 2005)). 14-3-3 dynamically binds proteins (including HERG) upon phosphorylation, primarily by PKA. When this occurs with HERG, channel activation is accelerated and current augmented. An LQT2 mutation has been described in which the deleterious effect is disruption of 14-3-3 binding (Choe et al., 2006). An A-kinase anchoring protein (AKAP) is likely involved in targeting PKA to HERG in a macromolecular complex, which may intensify current modulation (Li et al., 2008). The Kass group showed S27 in the KCNQ1 N-terminus is phosphorylated by PKA and this causes an increase in current. They also showed that a AKAP Yotiao targets PKA to the channel complex (Marx et al., 2002). These studies demonstrate an important, specific, and tightly controlled form of regulation by the components of the P-adrenergic pathway in relation to the two K+ channels.
In contrast to the P-adrenergic system, the a-adrenergic system involves phospholipase C, which hydrolyzes the membrane lipid phosphatidyl inositol-4,5-bisphosphate (PIP2) into the signaling molecules inositol 1,4,5-trisphosphate (IP3) and the second messenger diacylglycerol (DAG). DAG and calcium go on to activate protein kinase C (PKC) isoforms. An acute decrease in the PIP2 concentration, which occurs upon a-adrenergic stimulation, reduces HERG currents (Bian et al., 2001). This effect is dependent on consumption of PIP2 at the membrane and direct binding of PIP2 to HERG but occurs independently of calcium signaling or PKC activity (Bian et al., 2004; Bian & McDonald, 2007)). PKC regulation of HERG remains an active area of investigation where conclusive results await (Thomas, 2003) (Cockerill et al., 2007). For KCNQ1/KCNE1 and Iks, Varnum et al. showed that PKC stimulation decreased in IKs due to KCNE1 phosphorylation at serine-102 (Varnum et al., 1993). The mechanism of this IKs downregulation remained unclear until the Abbott group showed that PKC downregulates IKs current through inducing endocytosis (Kanda et al., 2011). Another group studied the regulation of IKs by PIP2 and showed that application of PIP2 delayed rundown of IKs in excised patch recordings (Loussouarn et al., 2003).
Correlation of mutational mechanisms with clinical phenotype and the approach to genetic testing results
Different channel mutations cause a range of clinical phenotypes, from very mild (asymptomatic) to severe (sudden cardiac death at a young age). Though some generalizations can be made correlating the mechanism by which a mutation acts and severity of clinical phenotype, the task is made difficult by the extensive list of implicated residues and their broad distribution across each gene. As one may expect, mutations to channel pore loops are generally severe, since they directly impact on channel conductance. A study of 858 LQT2 patients in 2009 revealed that patients with mutation to the pore region of HERG (S5 – pore loop – S6) had significantly higher rates of cardiac events than patients with mutations in the S1 – S4 transmembrane domains or the N- or C-termini, with the difference increasing with increasing age. The study also explored possible differences between types of mutations and found that in the C-terminus, patients with non-missense mutations were at significantly higher risk than those with missense mutations (Shimizu et al., 2009).
It is still difficult, though, to predict what type of cellular defect a certain mutation may case. Mutations that affect trafficking are not clustered in any particular region, and mutations that cause biophysical defects can also affect trafficking. K+ channel mutations are complicated by the ability to form wild-type/mutant heteromultimeric channels that exhibit different levels of defect depending on the number of mutant subunits. Functional analysis by in vitro expression of mutant channels is the only way to fully assess the cellular phenotype of a mutation. Additional genetic and environmental influences exist such that two patients with the same mutation may differ in clinical presentation. We do not yet know all the different factors that may affect the relative expression of mutant versus wild-type channels in a heterozygous patient, such that the distribution is not a 50/50 mix. One patient may express significantly differing amounts of normal or mutant allele subunits, and therefore have a variable clinical phenotype. For K+ channels, there is an overlap between the IKr and IKs currents in their role during repolarization (known as "repolarization reserve"), so the clinical presentation of a patient may be mild unless the unaffected current is also compromised by environmental factors.
A computational prediction tool called KvSNP for voltage-gated K+ channel genes to predict the severity of possible disease-causing mutations has been published (Stead et al., 2011). Two recent case studies illustrate the complexity of patient presentation and how prediction databases, although initially valuable, have limitations. Each case involved patients with QT prolongation noted on ECG, yet mild clinical history until presentation with sudden cardiac death in early adulthood. One patient had the LQT1 mutation KCNQ1-S277L, located in the S5 pore helix just proximal to the pore loop, predicted by KvSNP to be a severe mutation. The location suggests a biophysical defect, but thorough analysis revealed a combination of trafficking defect with a partially dominant-negative biophysical effect on heteromultimeric channels that managed to traffic properly to the membrane. The second patient had the LQT2 mutation HERG-G816V, located in the C-terminal region adjacent to a cyclic-nucleotide binding domain important for HERG regulation. This mutation was not predicted to be severe, yet functional analysis showed abnormal trafficking and significantly reduced current. Given the severe cellular defects, one would not predict a generally mild clinical phenotype. Both patients presented with sudden death when they experienced a second exogenous insult such as drug-induced blockade or electrolyte disturbance that reduced their remaining repolarizing current (Chen et al., 2011; Krishnan et al., 2011).
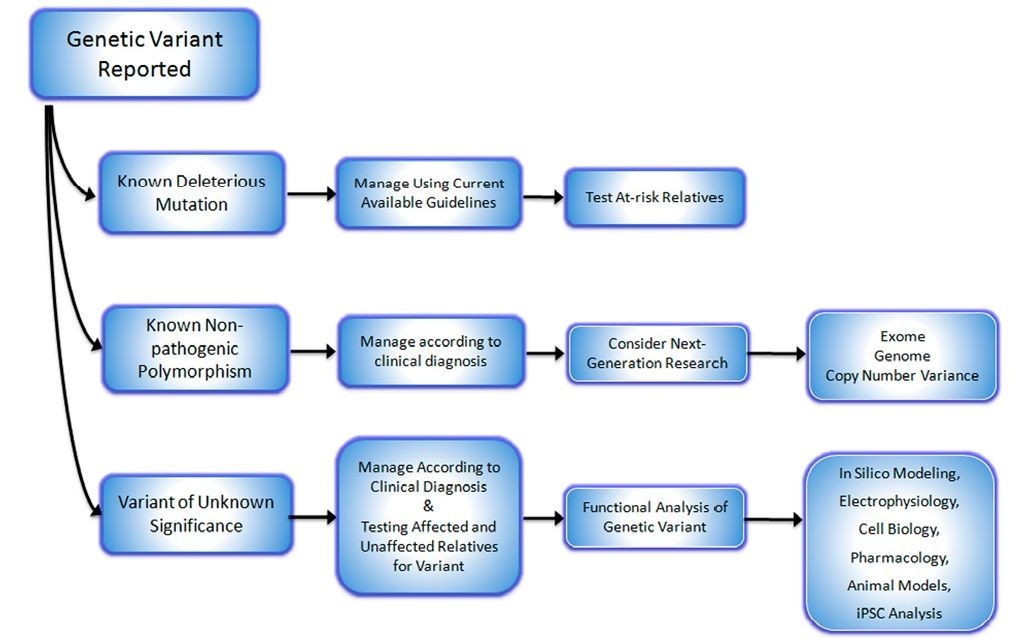
Fig. 2. A general approach to managing families with suspected hereditary arrhythmia syndromes after receiving genetic testing results.
These reports highlight the challenge that clinicians face upon receiving genetic testing results for patients suspected of having a hereditary cause of cardiac arrhythmia. The results from clinical laboratories may be given as clear-cut pathogenic deleterious mutations that have been reported in the literature. Such cases are relatively straightforward and further testing of at-risk family members is indicated with treatment and management dictated by the clinical presentation and recommendations for the documented mutation. Alternatively, the testing result may be read as a known non-pathogenic polymorphism that has been documented in normal populations. In this instance the clinician must guide therapy to the clinical diagnosis and consider whether the patient warrants further investigation such as analysis of copy number variance or various "omics" studies (whole exome or genome sequencing), which presently comprise investigative research studies. The third possibility is that genetic testing results are given as possible deleterious mutation or variants of unknown significance. This is a difficult puzzle for the clinician to solve. An initial step might be to search mutational or polymorphism genetic databases for reports of the given variant, but the commercial laboratories usually perform this task. Another is to submit the reported variant to in silico analysis as described above (KvSNP), but remaining aware of the potential inaccuracies. More desirable is to perform one or more of the several functional analyses outlined above. Although this will entail collaboration with an academic laboratory, it will provide more solid evidence for, or against the variant being deleterious. Figure 2 illustrates a suggested algorithm for the approach of genetic testing results.
Exploring novel therapeutic modalities
One of the greatest challenges in LQTS is developing new therapeutic modalities aimed at the root cause of the defect instead of managing or preventing arrhythmias. One example is designing methods that correct the trafficking defective phenotype in many LQT2 cases. Work by January and colleagues have sought to use pharmacological methods to rescue trafficking deficient HERG mutants (Gong, 2006). In some LQT2 mutations trafficking can be partially rescued in heterologous systems by lower temperature, glycerol or DMSO, which act as non-specific chaperones. HERG channel blocking drugs E-4031, astemizole and cisapride have also been shown to rescue some mutant-related trafficking defects, but functionality was abolished since the channel pore was blocked. As is the case for many in-vitro studies, the results are hard to translate into clinical therapies at present. Similar therapeutic models are in development for cystic fibrosis and rescue of CFTR trafficking mutants, but the same difficulties prevail (Becq et al., 2011). The ideal goal is to achieve a trafficking rescue without pore blockage. Encouraging results have been reported by Rajamani et al. who showed that the antihistamine fexofenadine was able to rescue some trafficking-deficient HERG mutants without channel block (Rajamani et al., 2002). Other efforts have utilized functional screens to discover small molecules that would suppress the long-QT phenotype irrespective of mechanism. An interesting approach has been reported using the breakdance (see section 4.3) mutant to screen for molecules that would rescue the phenotype (Peal et al., 2010). The investigators isolated 2 compounds that shortened the APD. The mechanisms by which these drugs work remain unclear as does the application of these drugs to mammals or later to humans. Nevertheless, this provides a good starting point and shows the utility of zebrafish as a genetic model in a high-throughput screen.
Conclusion
Modern medical genetics has advanced the diagnosis and treatment of hereditary arrhythmia syndromes greatly in the past 15 years. Future advances will include recognition of modifying genetic and environmental factors that influence penetrance and severity. There is also hope for novel gene- and mutation-specific therapies. An achievable goal in the sort-term will be clear delineation of genetic mutations and variants that presently reported to clinicians that patients and families with possible hereditary arrhythmias.