Introduction
It is well known that the heart generates and conducts electrical impulses, leading to a rhythmical contraction of the cardiac muscle. In normal situations, the atria contract about one sixth of a second ahead of ventricular contraction, allowing the filling of the ventricles before they pump the blood through the lungs and peripheral circulation. Additionally, all portions of the ventricles contract almost simultaneously, which is essential for a most effective pressure generation in the ventricular chambers. This rhythmical and conductive system is susceptible to damage by heart disease, especially by ischemia of the cardiac tissues. The result is often an abnormal heart rhythm and sequence of contraction of the heart chambers, leading to a reduction in pumping effectiveness, even to the extent of causing death (Hall, 2011).
Heart rate (HR) is not a static hemodynamic parameter but instead changes over time in response to physical and mental demands. HR is normally determined by spontaneous and periodic depolarizations of the sinoatrial node, the frequency of which is modulated by the sympathetic and parasympathetic divisions of the autonomic nervous system, the intrinsic cardiac nervous system, reflexes, and respiration. These neural systems also partially control cardiac contractility and conduction of electrical activity through the heart. As a result, HR (chronotropism), contractility (inotropism), and conduction (dromotropism) are adjusted to meet the changing needs of the body (Feldman et al, 2010).
Electrical activity of the heart
The properties of automaticity and rhythmicity are intrinsic to the cardiac tissue and considered a very complex phenomenon and, besides cellular mechanisms, integrative different factors are involved in cardiac pacemaking. The cardiac electrical events are initiated with changes in the permeability of the cell membrane, mainly to Na+, K+ and Ca2+ ions. Changes in cell membrane permeability alter the rate of ion passage across the membrane with the opening and closing of ion channels. Two main types of action potentials are observed in the heart: (A) fast action potentials, that occur in the normal myocardial fibers in the atria and ventricles and in the specialized conducting fibers (Purkinje’s fibers) and (B) slow action potentials, which are found in the sinoatrial (SA) node, the natural pacemaker of the heart, and in the atrioventricular (AV) node, the specialized tissue involved in conducting the cardiac impulse from atria to ventricles (Bouman and Jongsma, 1986).
In mammalian, the region of the heart that ordinarily generates impulses at the greatest frequency is the SA node. In humans, it lies in the groove where the superior vena cava joins the right atrium. It is a small, roughly rectangular region at the edge of the right atrium, bounded on two sides by the superior and inferior vena cava and on the other two by the interatrial septum and the crista terminalis, a part of the right atrial muscle over whose endocardial surface the pacemaking tissue of the SA node extends (Brown, 1982). The intact sinoatrial node is a heterogeneous structure and contains 2 principal types of cells: 1) small, round cells, which have few organelles and myofibrils; and 2) slender, elongated cells, which are intermediate in appearance between the round and the ordinary atrial myocardial cells. The round cells are probably the pacemaker cells, whereas the transitional cells probably conduct the impulses within the node and to the nodal margins (Verheijck et al., 2011; Verheijck et al., 2004; Tellez et al, 2006).
In the SA node cells, the upstroke of action potential is less steep, the plateau is not sustained and the depolarization is more gradual. However, the principal distinguish feature of a pacemaker resides in resting phase. In nonautonomic cells, the resting potential is constant, whereas in a pacemaker fiber there is as low depolarization that proceeds at steady rate until a threshold is attained, and then an action potential is triggered (Brown, 1982; Berne and Levy, 2009). In the pacemaker cells of the SA node the diastolic depolarization is attributed to at least 3 ionic currents: (1) an inward current (If), induced by hyperpolarization; (2) an inward Ca+2 current, (ICa); and (3) an outward K+ current, IK . The inward current (If) is carried mainly by Na+ and the current is conducted through specific channels that differ from the fast Na+ channels. This current becomes activated during the repolarization phase of the action potential, as the membrane potential becomes more negative than about -50mV. The more negative the membrane potential becomes at the end of repolarization, the greater is the activation of the If current. The second current responsible for diastolic depolarization is the slow inward current. This current is composed mainly of Ca+2 and therefore it is referred to as the Ca+2 current, (ICa). This Ca+2 current is carried mainly by T-type Ca+2 channels. Once the Ca+2 channels become activated, the influx of Ca+2 into the cell increases and accelerates the rate of diastolic depolarization, which then leads to upstroke of the action potential. The progressive diastolic depolarization mediated by the 2 inward currents, If and ICa, is opposed by a third current, an outward K+ current, IK . This efflux of K+ tends to repolarize the cell after upstroke of the action potential. The outward K+ current continues well beyond the time of maximum repolarization, but it diminishes throughout the end repolarization. Hence the opposition of IK to the depolarizing effects of the 2 inward currents (ICa and If) gradually decreases (Brown, 1982; Berne and Levy, 2009).
From the SA node the cardiac impulse spreads radially throughout the right atrium along ordinary atrial myocardial fibers, at a conduction velocity of approximately 1m/sec. A special pathway, the anterior interatrial myocardial band, conducts the impulse from the SA node directly to the left atrium. Tree tracts, the anterior, middle, and posterior internodal pathways, constitute the principal routes to the conduction of the cardiac impulse from the SA to AV node. The AV node contains the same two cell types as the SA node, however the round cells are sparser and elongated cells preponderate. The AV node has been divided into three functional regions: 1) the AN region, the transitional zone between the atrium and the remnant node; 2) the N region, the midportion of the AV node; and 3) the NH region, the upper portion of the specialized conducting system for the ventricles. Usually, the AV node and the bundle of His constitute the only pathways to action potential conduction from atria to ventricles. The conductive system passes subendocardially down the right side of the interventricular septum for about 1cm and divides into the right and left bundle branches. The right bundle branch is a direct continuation of the bundle of His and it proceeds down the right side of the interventricular septum. The left bundle branch, which is considerably thicker than the right, arises almost perpendicularly from the bundle of His and cross the interventricular septum. The right bundle branch and the two divisions of the left bundle branch ultimately subdivide into a complex network of conducting fibers called Purkinje’s fibers, which ramify over the subendocardial surfaces of both ventricles (Brown, 1982).
In the myocardium, the action potential generation includes 5 distinct phases: 1) Phase 0: the chemical and electrostatic forces both favor the entry of Na+ into the cell through fast Na+ voltage-gated channels to generate the upstroke; 2) Phase 1: the chemical and electrostatic forces both favor the efflux of K+ through transient outward current (Ito) channels to generate early, partial repolarization; 3) Phase 2: during the plateau, the net influx de Ca2+ through L-type Ca2+ voltage-gated channels is balanced by the efflux of K+ through rectifier (Ik), inwardly rectifying (Ik1) and Ito channels; 4) Phase 3: the chemical forces that favor the efflux of K+ through Ik, Ik1,Ito channels predominate over the electrostatic forces that favor the efflux of K+ through these same channels; 5) Phase 4: the chemical forces that favor the efflux of K+ through Ik and Ik1 channels exceed very slightly the electrostatic forces that favor the influx of K+ through these same channels (Berne and Levy, 2009).
Neural control of HR
The peripheral circulation distributes the cardiac output to the various organs and tissues according to their individual metabolic or functional needs while maintaining arterial blood pressure within a relatively narrow range. Regional blood flows can be efficiently regulated at the local level by the intrinsic ability of vessels to respond to various mechanical forces (e.g., wall tension and shear stress) as well as chemical stimuli (e.g., tissue metabolites and O2). However, a perfect regulation of the peripheral circulation cannot be achieved only by the local vascular control mechanisms, but require the coordinating activity of central neural outflow to the heart and blood vessels (Thomas, 2011). In this field, the autonomic nervous system plays an important role to normal cardiovascular control and changes in autonomic balance has been related to several cardiovascular disorders, such as cardiac arrhythmias and hypertension (Workman, 2010; Pagani and Lucini, 2001).
The autonomic nervous system and cardiovascular control
The autonomic nervous system is responsible for the involuntary control of most visceral organs, including the heart and the interactions between the sympathetic and parasympathetic limbs play a critical role in cardiac electrical stability and arrhythmias generation. In general, sympathetic activation has a profound arrythmogenic potential (Schwartz et al., 1978, Schwartz 1984). Experimental stimulation of sympathetic nerves or stellate ganglia induces ECG repolarization changes and reduces the fibrillation threshold, facilitating ventricular fibrillation (Yanowitz et al, 1966; Podrid et al, 1990), while the use of P-adrenergic blocking agents can improve survival in patients following myocardial infarction (Gottlieb et al, 1998). On the other hand, vagal activation has a powerful antifibrillatory effect (Vanoli et al., 1991; De Ferrari et al., 1994). Therefore, autonomic imbalance could become either proarrythmic or anti-arrythmic based on which of the two components is going to prevail (Schwartz and De Ferrari, 2011).
Preganglionic fibers of autonomic nervous system are originated from central nervous system (CNS) at the level of the brainstem or sacral spinal cord (parasympathetic fibers) and the thoracic or lumbar spinal cord (sympathetic fibers). Both parasympathetic and sympathetic preganglionic fibers release acetylcholine which binds to nicotinic receptors located in the cell bodies of postganglionic neurons, leading to action potential generation. This synapse occurs in autonomic ganglia located outside of the CNS (Thomas, 2011). The axons of postganglionic neurons innervate the effector tissues, including cardiovascular tissues. Parasympathetic neurons are distributed much more heterogeneously throughout the heart than sympathetic neurons. The density of parasympathetic innervation in the sinoatrial (SA) and AV nodes is considerably higher than in the surrounding atrial or ventricular tissue (Vaseghi and Shivkumar, 2008). Cardiac sympathetic innervation of the heart includes innervation of the SA node and myocardial cells. Based on norepinephrine content studies, a gradient exists in sympathetic innervation from atria to ventricles and from base to apex of the heart, indicating that the atria are most densely innervated, but the ventricles are also supplied with a sympathetic network, most densely at the base (Vaseghi and Shivkumar, 2008). Regarding the neurotransmitters, postganglionic parasympathetic fibers release acetylcholine, which binds to muscarinic receptors on the target tissue, while postganglionic sympathetic fibers release norepinephrine, which binds to either a or p adrenergic receptors (Thomas, 2011).
The effects of sympathetic and parasympathetic neurons on HR will be based on changes in the ion currents of SA node action potential generation. Norepinephrine release from post ganglionic sympathetic neurons will increase the slope of diastolic depolarization in SA node by the enhancement of the resting potential, while acetylcholine release from parasympathetic postganglionic neurons will decrease the slope of diastolic depolarization by hyperpolarization of the resting potential (Verrier and Tan, 2009). Additionally, sympathetic stimulation increases the rate of conduction as well as the level of excitability in all portions of the heart and augments greatly the force of contraction of all the cardiac musculature. Maximal stimulation can almost triple the frequency of heartbeat and can increase the strength of heart contraction as much as twofold. On the other hand, parasympathetic stimulation to the heart decreases the excitability of the A-V junctional fibers between the atrial musculature and the A-V node, thereby slowing the transmission of the cardiac impulse into the ventricles (Guyton and Hall, 2006).
Given the ability to modulate both HR and stroke volume, the autonomic nerves provide an important mechanism to rapidly adjust cardiac output to meet short-term changes in the body’s needs (cardiovascular reflexes). In humans, there is a good deal of tonic vagal discharge and a moderate amount of tonic sympathetic discharge, showing a parasympathetic prevalence on the heart. Additional vagal discharge can further reduce HR, consequently cardiac output, whereas additional sympathetic discharge can increase HR and stroke volume and augment cardiac output. Conversely, withdrawal of tonic vagal or sympathetic discharge has opposing effects to increase or decrease cardiac output, respectively (Thomas, 2011).
Cardiovascular reflexes
It is well known that the maintenance of arterial pressure at adequate levels to perfuse the tissues is a basic requirement for survival. In cardiovascular system, among the mechanisms that act buffering arterial pressure fluctuations we can highlight the role of the neural reflexes. Such control is an important pathway to effect rapid changes in blood pressure and in the distribution of cardiac output that are essential to maintain a sufficient perfusion to vital organs, such as heart, brain and the kidney in face of physiological and environmental challenges. This rapid control of cardiovascular function is achieved through arterial and non-arterial reflexes that detect and correct changes in arterial blood pressure (baroreflex), blood volume (cardiopulmonary reflex) or chemical composition (chemoreflex) of the blood (Vasquez et al., 1997). It is important to notice that the effectiveness of these systems may be modulated by hormonal systems, such as angiotensin II and nitric oxide. The understanding of the key concepts about these reflexes under physiological conditions and the effects of hormonal substances on its functioning is an important step to clarify the development of arrhythmias.
Baroreflex
The baroreflex feedback loop is one of the most important mechanisms controlling arterial pressure. The main purpose of the baroreflex function is to provide a rapid and efficient stabilization of arterial blood pressure on a beat-to-beat basis by means of strategically located arterial sensors which are sensitive to high blood pressure and known as arterial baroreceptors. The baroreceptors endings are located in adventitia layer of carotid sinus and aortic arch with their soma located in the petrosal and nodose ganglia respectively. These receptors are mechano-sensitive and the distension of the vessels that occurs at each heart beat leads to action potential generation on these terminals which are transmitted to CNS, buffering arterial pressure fluctuations through changes in sympathetic and parasympathetic activity (Vasquez et al., 1997).
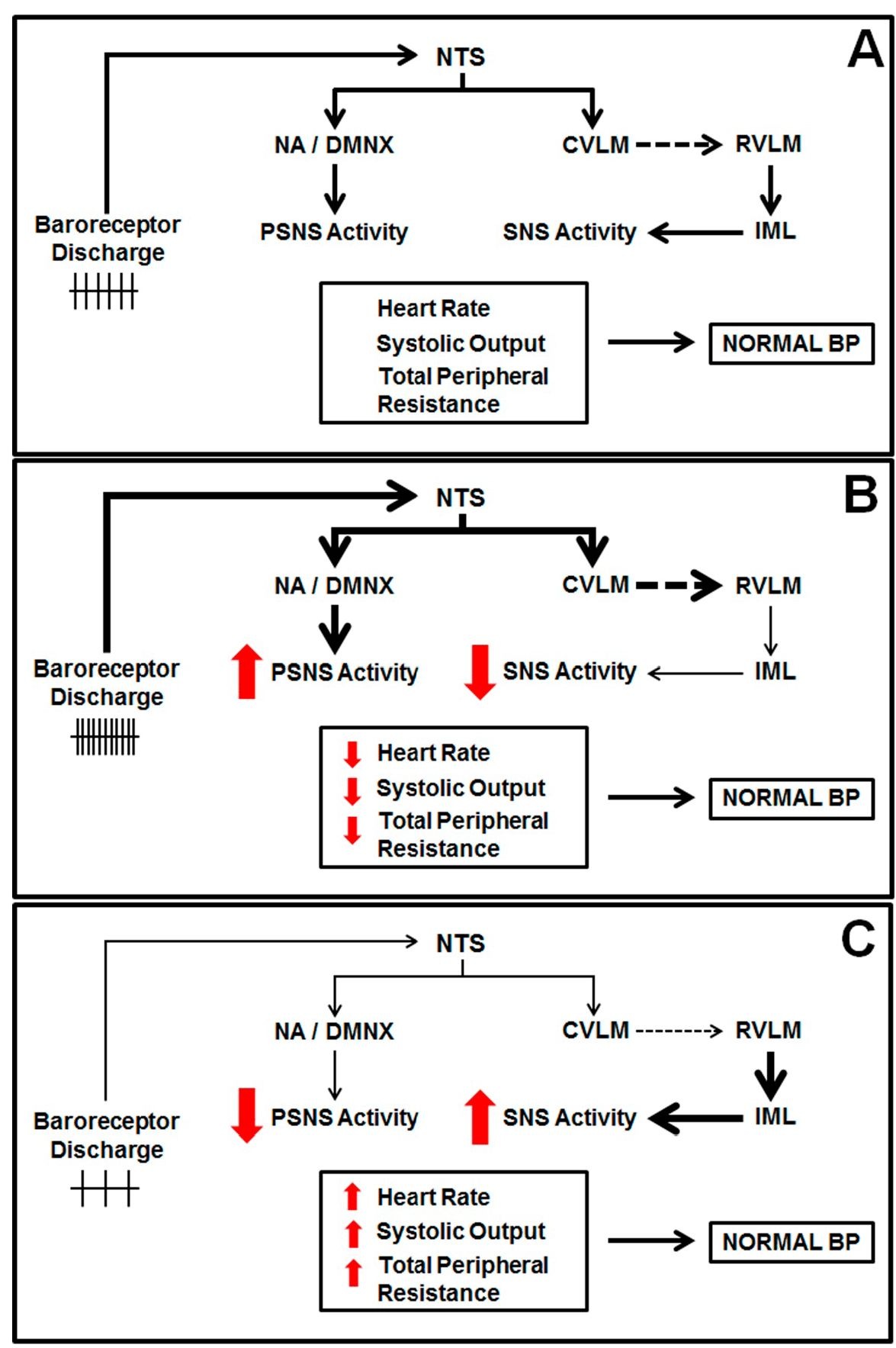
To achieve this precise control, the generated action potentials in each systole travel centrally to synapse onto neurons in the nucleus tractus solitarii (NTS) in the dorsal medulla. NTS neurons project to "higher" brain nuclei, as well as other nuclei in the brainstem that are critical for efferent sympathetic and parasympathetic activity (Loewy and Spyer, 1990). Projections from NTS are connected to the inhibitory neurons of caudal ventrolateral medulla (CVLM) that subsequently synapse to excitatory neurons in the rostral ventrolateral medulla (RVLM). RVLM exerts a tonic discharge upon the preganglionic sympathetic neurons, located in the intermediolateral column (IML) of the spinal cord (Kirkman and Sawdon, 2004). Therefore, activation of the baroreceptor afferents innervating the NTS causes excitation of neurons projecting to the CVLM, which in turn inhibits RVLM. These events lead to less activity from the RVLM to IML, reducing sympathetic efferent activity (Figure 1A). Several studies have demonstrated that disturbances in the normal functioning of these nuclei can be related to the development of arrhythmias. In example, Issa et al. (2005) showed that the central inhibition of the sympathetic drive using clonidine reduces the occurrence of ventricular tachycardia/ ventricular fibrilation in a canine heart failure model.
In parallel, NTS neurons also synapse onto preganglionic vagal neurons localized within nucleus ambiguus (NA) and in the dorsal motor nucleus of the vagus (DMNX, Figure 1A). These neurons dominate the neural control of HR under normal conditions and also influence the prognosis of many cardiovascular disorders, such as sudden cardiac death, ventricular fibrillation, and myocardial ischemia (Wand et al, 2001). The axons from preganglionic cardiac vagal neurons travel down the vagus nerve and synapse onto postganglionic cardiac vagal neurons in cardiac ganglia. The synaptic innervation of cardiac vagal neurons is therefore critical for the tonic and reflex evoked changes in cardiac vagal activity that control HR.
Fig. 1. Schematic diagram showing the baroreflex functioning during normal (A), increased (B) and decreased blood pressure (C). NTS: nucleus tractus solitarii, NA: nucleus ambiguus, DMNX: dorsal motor nucleus of the vagus, CVLM: caudal ventrolateral medulla, RVLM: rostral ventrolateral medulla, IML: intermediolateral column, PSNS: parasympathetic nervous system, SNS: sympathetic nervous system, BP: blood pressure. The continuous arrow and the dashed arrows indicate a stimulatory and an inhibitory synapse, respectively.
Therefore, when blood pressure rises, the baroreceptor afferent activity augments, leading to increased vagal activity and diminished sympathetic outflow. These effects will reduce HR and cardiac contractility, causing a decrease in cardiac output. Additionally, the fall the sympathetic activity to blood vessels also leads to vasodilation, diminishing the vascular resistance (Figure 1B). The reduced cardiac output and vascular resistance return blood pressure to its original level. On the other hand, a fall in blood pressure results in reduced baroreceptor afferent activity, causing a decrease in vagal activity and augmented sympathetic outflow (Figure 1C). These events increase cardiac output and vascular resistance, normalizing arterial blood pressure.
Experimentally, the baroreflex function can be evaluated through changes in arterial pressure. In bolus phenylephrine injections elicits increases in arterial pressure leading to reflex bradycardia and sodium nitroprusside injections reduces arterial pressure causing reflex tachycardia. Typical recordings of baroreflex evaluation are displayed in Figure 2.