Anatomical peculiarity of hibernator’s heart
An insight into the anatomy of a hibernator’s heart may provide clues as to how a hibernator can overcome heart failure under extreme hypothermia. The peculiar anatomy of the heart of a hibernator has been described by Walls in a hamster (Walls, 1942). Several interesting features of the conducting tissue have been identified in this study. Purkinje fibers are identified in the sino-atrial node, the pacemaker of the heart, and not in the atria suggesting that Purkinje fibers may have a function other than a simple conduction of the cardiac contraction impulse. The atrio-ventricular node has a compound nature of fibers which are similar to Purkinje type. Purkinje tissue is absent in the right ventricle and a limited amount of Purkinje tissue is present in the left ventricle whose wall is six times thicker than the right ventricle. In spite of limited Purkinje tissue distribution to the ventricles it is interesting to note that the heart is capable of about 450 beats per minute.
Gap junctions are specialized intercellular connections in the heart and are needed for conduction in the heart. Gap junctions ensure the propagation of action potentials between the myocytes and provide low resistance intercellular channels facilitating coordinated contraction of myocardium (Saitongdee et al., 2000). Connexins are gap junction proteins with four-membrane spanning domains. Among several types of connexins, connexin43 (Cx43) is the major connexin found in the mammalian heart (Beyer et al., 1987). Cx43 and Cx45 are upregulated in the hearts of hibernators (Gros and Jongsma, 1996; Fedorov et al., 2005; Van Der Heyden et al., 2007). Increased density of Cx43 has been identified in ventricular cardiomyocytes of hibernators during hibernation (Saitongdee et al., 2000). Cx43 density returned to normal control levels within 2 hours of arousal from torpor suggesting the importance of Cx43 and Cx45 in overcoming ventricular fibrillation during hibernation. (Saitongdee et al., 2000; Fedorov et al., 2005).
Adenosine in hibernation
A growing body of evidence supports the significance of adenosine in hibernation (Drew et al., 2007). Adenosine is a widely distributed inhibitory neuromodulator throughout the central nervous system including the brainstem, the principle cardiovascular control center (Mosqueda-Garcia et al., 1989; Barraco and Phillis, 1991). Adenosine decreases neuronal excitability and modulates the actions of other neurotransmitters (Dunwiddie and Masino, 2001). Adenosine acts through A1, A2a, A2b, and A3 receptors (Fredholm et al., 1994; Olah and Stiles, 1995; Dunwiddie and Masino, 2001). Endogenous adenosine is produced from multiple sources in the central nervous system, some sources associated to energy levels and functions as a homeostatic regulator in the CNS (White, 1977; Fredholm et al., 1994; Dunwiddie and Masino, 2001). Dephosphorylation of adenosine triphosphate (ATP) is one of the major sources of endogenous adenosine production where ATP released into synapse is metabolized to adenosine and mediates its effect through adenosine receptors (Fredholm et al., 1994; Dunwiddie and Masino, 2001).
Adenosine in induction of torpor during hibernation
Central nervous system regulation of hibernation is implicated by several studies (Drew et al., 2007; Jinka et al., 2011). Recent study has shown that administration of the adenosine A1 receptor agonist N6-cyclohexyladenosine (CHA) into the lateral ventricle of arctic ground squirrel induces hibernation. CHA-induced hibernation is similar to natural spontaneous entrance into hibernation. Results indicate that onset of hibernation is regulated within the central nervous system through activation of A1AR (Jinka et al., 2011). Studies focusing on specific sites in the brain including the hypothalamus and hippocampus indicate a prominent influence of CNS on hibernation (Heller and Colliver, 1974; Popov et al., 1992). Studies on central nervous system also direct towards involvement of adenosine, a neuromodulator in hibernation regulation (Shintani et al., 2005; Tamura et al., 2005; Jinka et al., 2011).
Successful translation of hibernation to non-hibernating species will open possibilities of applying the concept of metabolic suppression and low body temperature to humans in treating conditions such as stroke, hemorrhagic shock, cardiac arrest, cerebral ischemia, and multiorgan failure (Drew et al., 2007).
Significance of adenosine on dietary restriction induced hypothermia and cardiovascular regulation
Adenosine-induced hypothermia is mediated through A1AR (Dunwiddie and Masino, 2001; Shintani et al., 2005). Adenosine modulates the cardiovascular system through numerous A1AR in nucleus tractus solitarius (NTS) (Badman and Flier, 2005; Scislo et al., 2008) located in the brainstem which receives projections from hypothalamus, the thermoregulatory center in the brain (Scislo and O’Leary, 2006). Cardiovascular centers of the medulla are innervated by projections from the hypothalamus which alleviates cardiac arrhythmias by modulating the blood pressure (Willette et al., 1984; Lumb and Lovick, 1993; Kiely and Gordon, 1994; Hirasawa et al., 1996; Krukoff et al., 1997; Yang and Coote, 1998; Hardy, 2001). NTS influences cardiovascular system. Hypotensive responses in the cardiovascular system are mediated through A1AR in NTS (White et al., 1996). Adenosine microinjections into the NTS result in a slow and regulated decrease in heart rate (Tseng et al., 1995; Phillis et al., 1997; Ho et al., 2008). Thus NTS and A1AR contribute significantly towards induction of hypothermia and modulation of cardiovascular responses.
Dietary restriction is a dietary regimen defined by a decrease in food intake unassociated with malnutrition which lowers core body temperature, improves longevity, protects heart and attenuates progression of neurodegenerative diseases in animal models (Contestabile, 2009; Katare et al., 2009). These effects have been suggested to be through a reduction in metabolic demand (Ungvari et al., 2008) associated with a decrease in body temperature (Tb)(Conti et al., 2006). Mechanisms involved in induction of hypothermia are under investigation. Results from our studies have shown that DR-induced hypothermia is due to adenosine sensitization (Jinka et al., 2010). Our results have demonstrated that intraperitoneal administration of CHA (0.5mg/kg) in DR-sensitized rats induced a significant cooling undetected in ad libitum (AL) rats. However, it is not clear as to how the heart responds to this induced cooling in DR rats because hypothermia beyond a certain level is not without complications like cardiac arrhythmias (Polderman and Herold, 2009). It was shown that DR has certain beneficial effects on heart (Lee et al., 1999) including protection from arrhythmias (Johnson et al., 2006) although it is yet to be investigated whether these beneficial effects on heart are applicable under hypothermic conditions induced by the A1AR agonists.
Central administration of A1AR agonist-induced hypothermia in Syrian hamsters is free of cardiac arrhythmias while forced induction of hypothermia through intraperitoneal pentobarbital sodium causes J-waves and atrioventricular block (Miyazawa et al., 2008). Syrian hamsters undergo periods of food restriction, a process comparatively similar to dietary restriction, which prepares them to hibernate (Stamper et al., 1999). Dietary restriction influences NTS (Badman and Flier, 2005). Thus it can be hypothesized that centrally administered A1AR agonist-induced hypothermia in dietary restricted rats may avoid cardiac arrhythmias.
Previous studies and results
In our previous studies we have shown that prolonged DR sensitizes A1AR agonist-induced cooling. Sprague-Dawley rats were implanted with subcutaneous IPTT-300 transponders for monitoring body temperature. Rats were fed every other day for 27 days and then administered the A1AR agonist, N6-cyclohexyladenosine (CHA; 0.5mg/kg, ip). Respiratory rate (RR) and subcutaneous body temperature were monitored every day and after drug administration. A lower RR on day 20 and lower body temperature on day 22 were displayed by DR rats when compared to rats fed ad libitum and displayed a larger response to CHA. RR, a metabolic indicator, declined before body temperature in all cases suggesting that a decrease in oxidative metabolism associated with thermogenesis caused animals to cool. This is comparable to torpor because of prior changes in metabolism than body temperature as observed during hibernation (Lyman, 1958). An increased surface expression of A1AR is demonstrated within the hypothalamus in DR rats. These results suggest that sensitization of thermoregulatory effects of endogenous adenosine through increased surface expression of A1AR may play a role in enhanced hypothermia associated with DR. These results also suggest that a torpid like effect is seen with CHA-induced hypothermia in DR rats. However, it is not known from these studies as to how the heart responds to this CHA- induced hypothermia in DR rats (Jinka et al., 2010).
Hypothermia in hibernation vs hypothermia in A1AR stimulated DR rats
Hypothermia is seen in hibernators during torpor where their core body temperature (Tb) can reach to as low as -2.9°C (Barnes, 1989) without any complications. A sudden drop in metabolism followed by a decrease in core body temperature is the hallmark of hibernation (Lyman, 1958). CHA-induced hypothermia in DR rats resembled torpor in hibernators as there is a sudden decrease in respiration, an indicator of metabolism, followed by a slow decrease in body temperature (Jinka et al., 2010). Central administration of CHA in hibernators results in hypothermia without any untoward effects on heart while cardiac arrhythmias were seen with anesthetic-induced hypothermia (Miyazawa et al., 2008). Atrioventricular blocks and J-waves are observed in nonhibernators during induced hypothermia (Osborn, 1953; Brunson et al., 2005). Appearance of J-waves, also known as Osborne waves, indicates injury, delayed ventricular conduction, tissue anoxia or acidosis (Miyazawa et al., 2008). These studies suggest that an unidentified intrinsic mechanism in the heart of hibernators may be responsible for circumventing heart failure under extreme hypothermia.
Neuroprotection by induction of hypothermia and circumventing cardiac arrhythmias
Neuronal cell death is one of the major aftermaths of cardiopulmonary arrest and stroke. Under clinical setting, regulated hypothermia induced in the stroke patient in order to mitigate neuronal injury has proven to be helpful. Neuroprotection is evident in hibernators which experience extreme hypothermia. Thus inducing a hibernation-like state would be more beneficial in cardiac arrest patients. DR-induced cooling is well established in various rodents (Conti et al., 2006; Ungvari et al., 2008; Contestabile, 2009). What is novel in the recent research is that adenosine A1 receptor (A1AR) agonist; CHA administration induces increased hypothermic response in DR rats (Jinka et al., 2010), although the response of the cardiovascular system is not measured. This CHA-induced hypothermia in DR rats is similar to the torpor seen in hibernation and this is achieved through sensitization of A1AR in the brain’s hypothalamus, the principle thermoregulatory center in the CNS. Recent study in hibernators also has shown that central administration of CHA induces cooling without cardiac arrhythmias (Miyazawa et al., 2008). Hence there is a possibility of circumventing cardiac arrhythmias in DR rats when hypothermia is achieved through central administration of CHA. Thus it can be hypothesized that A1AR agonist-induced hypothermia in dietary restricted rats may avoid cardiac arrhythmias.
Hypothesized model
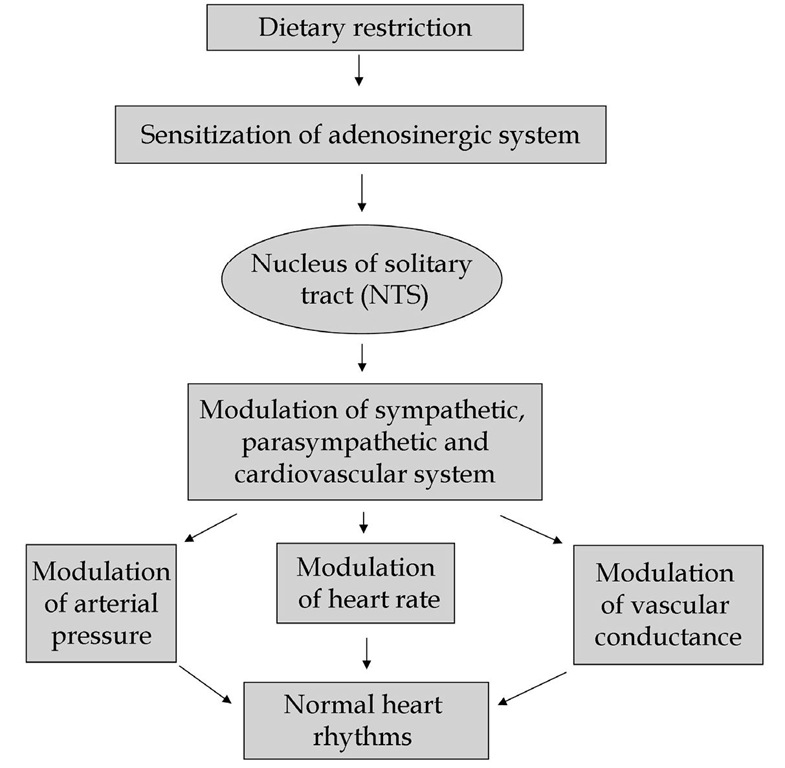
Sensitized adenosinergic system in DR rats acts through nucleus of the solitary tract (NTS), a primary integrative center for cardiovascular reflex. Adenosine in NTS modulates sympathetic, parasympathetic, and cardiovascular systems which in turn modulate arterial pressure, heart rate and vascular conductance by acting on and tuning the activity of the sympathetic and parasympathetic systems. The effect of adenosine may be one of the mechanisms behind cardioprotective effect (Fig.4) leading to generation of normal cardiac rhythms circumventing cardiac arrhythmias.
Fig. 4. Hypothesized model of dietary restriction induced cardioprotection.
Conclusion
Hibernators undergo a variety of complex morphological, behavioral, and physiological adaptive changes during hibernation period. Profound metabolic suppression, hypothermia, and bradycardia observed at the organismal level during the hibernation period have no harmful effects (Geiser, 1988; Barnes, 1989; Buck and Barnes, 2000; Drew et al., 2001; Zhou et al., 2001; Carey et al., 2003a; Heldmaier et al., 2004; Tamura et al., 2005; Ross et al., 2006; Drew et al., 2007). The hearts of hibernating mammals remain functional even at 0°C while the hearts of non-hibernating mammals becomes arrhythmic and stop functioning between 10°C and 15°C (Lyman, 1982; Caprette and Senturia, 1984; Burlington and Darvish, 1988). This implies that an intrinsic difference in functional mechanism may exist between the hearts of a hibernator and a non-hibernator enabling the hibernator to survive despite low body temperatures. Understanding the mechanisms regulating hibernation has the potential to develop therapies for conditions such as cardiac arrhythmias, hemorrhagic shock, stroke, cardiac arrest and cerebral ischemia (Drew et al., 2007).