Introduction
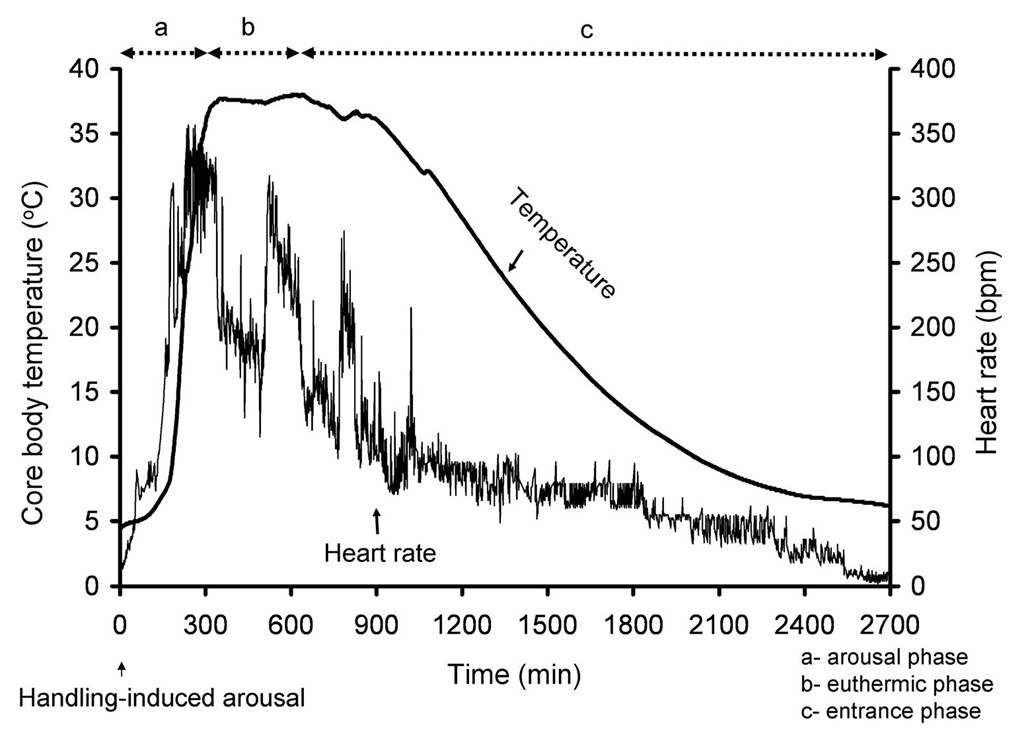
Hibernation is a physiological adaptation to periods of seasonal resource limitation (Carey et al., 2003a; Drew et al., 2007). Hibernators undergo several bouts of torpor during a hibernation season. Torpor in hibernation is a period of profound bradycardia, tachycardia, metabolic suppression and decreased core body temperature (Drew et al. 2007). Hibernation is characterized by alternating phases of torpor and euthermy that begins in the fall and continues until the hibernation season ceases in spring (Lyman, 1958; Geiser and Ruf, 1995; Boyer and Barnes, 1999). Based on whole-body metabolic rate and core body temperature each torpor bout consists of an entrance, steady-state, and arousal phases (Boyer and Barnes, 1999; Carey et al., 2003a; Heldmaier et al., 2004; Drew et al., 2007) (Fig.1). Successive torpor bouts are interrupted by a brief period (12-24h) of interbout euthermy.
Cardiac arrhythmia is described as any deviation from the normal sequence of electrical impulses resulting in slow (bradycardia), fast (tachycardia) or erratic hearbeats such as atrial and ventricle fibrillations and conduction disorders (Keating and Sanguinetti, 2001). Cardiac arrhythmias are observed during hibernation (Chatfield and Lyman, 1950; Eagles et al., 1988; Milsom et al., 1993; Milsom et al., 1999; Toien et al., 2011). In spite of that no untoward effects such as ventricular fibrillation or heart failure are noticed in hibernators and the hearts remain functional even at a body temperature of 0oC (Johansson, 1996). Moreover, hibernators can rewarm to euthermic body temperature of about 36oC in a span of few hours (Lyman, 1958)(Fig1&2) without any cardiac or nervous system complications (Drew et al., 2007). In contrast, similar conditions in non-hibernators including humans lead to fatal cardiac complications and death (Nardone, 1955; Johansson, 1996; Drew et al., 2007). Unresolved intrinsic mechanisms protect the hibernating species against lethal cardiac arrhythmias at reduced body temperatures. Understanding the intrinsic functional mechanisms existing in hibernators can lead to novel therapies in treating several conditions such as cardiac arrest and stroke (Drew et al., 2007).
Patients with cardiac arrest are subjected to hypothermia in a clinical setting (Polderman, 2004; Polderman and Herold, 2009). However, inducing hypothermia beyond a certain level is not without complications. Patients subjected to temperatures colder than 30oC suffer cardiac arrhythmias (Polderman and Herold, 2009). Cooling more slowly should mimic similar drop in body temperature seen during torpor in hibernators and may thus avoid arrhythmias. The question is how to achieve this state where the temperature can be dropped below 30oC without any cardiac complications.
Difference exists between hibernators and nonhibernators in resisting ventricular fibrillation induced by hypothermia. Several factors are responsible including heart size (Surawicz, 1971).Hibernating animals vary in size (Geiser, 2004). Large hearts tend to develop ventricular fibrillations (Surawicz, 1971). Although bears are regarded as hibernators their body temperature does not fall below 30oC which is above the critical body temperature where ventricular fibrillations are noticed (Johansson, 1984; Eagles et al., 1988; Toien et al., 2011). This topic discusses about small hibernators in general focusing on the role of nervous system regulation of cardiac function in the light of recent research findings and the importance of adenosine (Miyazawa et al., 2008; Jinka et al., 2011). This topic gives an overview of hibernation physiology, various mechanisms regulating hibernation, cardiac arrhythmias observed during hibernation, functional difference between hibernator and a non-hibernator, especially in regard to heart function, and finally discusses novel findings and hypothesis that may be translated to treat certain medical conditions such as cardiac arrest and stroke to improve the outcome in such patients.
Phases of hibernation and cardiac arrhythmias
The entrance phase
A decrease in heart rate and metabolism prior to decrease in core body temperature is a characteristic phenomenon observed during entrance into hibernation (Lyman, 1958). Heart rate, metabolism and core body temperature gradually decline during the entrance phase until the core body temperature drops down to the lowest limit where the core body temperature is just above the ambient temperature (Boyer and Barnes, 1999; Tamura et al., 2005). Heart rate declines to 2-7 beats per minute (Dawe and Morrison, 1955) , metabolism drops to 2% of resting metabolic rate (Geiser, 1988; Buck and Barnes, 2000) and core body temperature drops to as low as -2.9o C (Barnes, 1989)(Fig.1&2).
Cardiac arrhythmias during entrance into hibernation
Evidence supports the central nervous system regulation during entrance into hibernation. Administration of adenosine agonist into the brain induces torpor in arctic ground squirrels (Jinka et al., 2011). By lowering the set-point (Tset) threshold below the actual hypothalamic temperature (Thy) during entrance into hibernation a smooth entrance is facilitated. An occasional burst of body temperature paralleled by an increase in metabolism is observed when Thy below Tset. (Heller et al., 1977; Heldmaier et al., 2004). The changes in heart rate parallel the change in metabolic rate suggesting that entrance into hibernation is a highly regulated, orchestrated event of several physiological processes rather than a consequence of a drop in body temperature (Milsom et al., 1999).
A comparison between heart rate and temperature in hedgehogs during entrance into hibernation indicates a shift towards parasympathetic influence (Dawe and Morrison, 1955). Atropine is a parasympatholytic and increases heart rate by slowing of parasympathetic output. Administration of atropine during entrance into hibernation increased heart rate in hamsters (Lyman and O’Brien, 1963). Cardiac arrhythmias during entrance into hibernation are abolished by administration of atropine in marmots (Lyman, 1982). All these studies suggest that a well coordinated activation of parasympathetic system, preparatory initial changes in the heart rate, skipped beats and asystoles altogether are necessary for decline in heart rate and for a smooth entrance into hibernation (Milsom et al., 1999; Zimmer et al., 2000).
Fig. 1. Core body temperature was measured in an Arctic ground squirrel after arousal from torpor induced by gentle handling and until the animal entered another bout of torpor.
Core body temperature and heart rate were measured with an ip transmitter as described previously (Jinka et al., 2011). Torpor in hibernation is broadly divided into three phases-entrance, steady-state, and arousal (Boyer and Barnes, 1999; Carey et al., 2003a; Heldmaier et al., 2004; Drew et al., 2007). Entrance phase is followed by steady-state phase which lasts for 1-3 weeks before the arousal phase is initiated. Core body temperature in an Arctic ground squirrel can drop to as low as -2.9oC (Barnes, 1989) before it reaches steady-state phase. A fully aroused animal stays at euthermic body temperature of 35-37oC for about a day before another torpor bout ensues. Changes in heart rate reflect changes in core body temperature during a hibernation bout.
Several unique behavioral patterns of heart beats are noticed during entrance into hibernation. It is interesting to know how a hibernator can drastically reduce its heart rate during entrance into hibernation without any adverse effects. Heart rate drops prior to any changes in body temperature indicating that a decreased heart rate during entrance into hibernation is independent of body temperature. (Landau and Dawe, 1958; Lyman, 1958; Elvert and Heldmaier, 2005). Appearance of skipped beats is a characteristic feature exhibited by several species of hibernators during entrance into hibernation (Dawe and Morrison, 1955; Lyman, 1958; Twente and Twente, 1978; Lyman, 1982) (Fig.3). A drastic 50% fall in heart rate while a drop in body temperature by 0.6oC observed during initial stages of entrance into hibernation occurs around 33-34oC (Strumwasser, 1959).
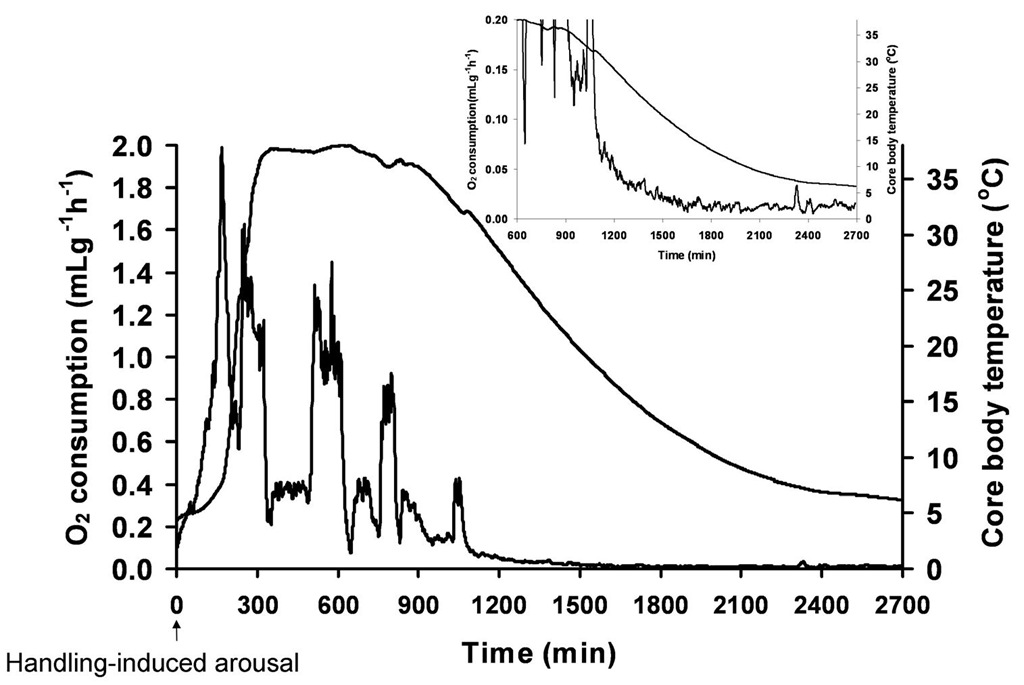
Fig. 2. Changes in core body temperature and whole animal oxygen consumption as measured in an Arctic ground squirrel after arousal from torpor was iniated by gentle handling and until the animal entered another bout of torpor.
Core body temperature was measured with an ip transmitter and oxygen consumption was measured using open flow respirometry as described previously (Jinka et al., 2011). The inset illustrates how oxygen consumption precedes a decline in core body temperature during entrance into torpor as shown on a smaller scale. Entrance into hibernation is characterized by a characteristic decline in whole animal oxygen consumption (metabolism) that precedes a decrease in core Tb (Lyman, 1958). [source: (Drew et al., 2009)]
The steady-state phase
Animal enters into a steady-state phase of hibernation after a few hours of initiation of torpor. Steady-state phase represents the nadir of mammalian heart rate, metabolism, and core body temperature (Drew et al., 2007) where the animal maintains its lowest heart rate, metabolism, and core body temperature for about 1-3 weeks (Boyer and Barnes, 1999; Buck and Barnes, 2000; Carey et al., 2003a). An occasional burst of activity paralleled with an increase in heart rate, metabolism and heat production is observed during this phase and is hypothesized as a measure to avoid decreases in body temperature beyond a certain point (Heldmaier et al., 2004).
Cardiac arrhythmias during steady state hibernation
Diastolic arrhythmias are noticed in deep hibernation (Twente and Twente, 1978; Milsom et al., 1999). Different opinions exist on the influence of sympathetic and parasympathetic systems on deep hibernation with no definitive conclusion (Milsom et al., 1999).
The arousal phase
Periodic arousals from hibernation are noticed in true hibernators (Lyman, 1958; Geiser and Ruf, 1995; Boyer and Barnes, 1999; Karpovich et al., 2009). A characteristic gradual increase in heart rate, metabolism and respiration followed by a gradual increase in core body temperature is observed during arousal from hibernation (Lyman, 1958). It is interesting to note that the rewarming from hibernation without any external source of heat suggests that hibernation is not a state of energy deficiency (Carey et al., 2003a). Animals attain a core body temperature of 35-37oC, then maintain euthermic body temperature for about a day before another hibernation bout starts (Boyer and Barnes, 1999; Carey et al., 2003b).
Cardiac arrhythmias during arousal from hibernation
Cardiac arrhythmias appear throughout arousal (Twente and Twente, 1978). Heart rate gradually increases in frequency as the body temperature increases (Lyman, 1958). The initial rapid increase in heart rate during arousal from hibernation is due to sympathetic activation (Milsom et al., 1993) and as such the increase in endogenous catecholamines are arrhythmogenic (Burn, 1961; Trautwein, 1963). Asystoles are followed by bradycardia during arousal from hibernation. Asystoles appear between 11-18oC during which period the heart rate falls below what it was before the appearance of asystolic episodes, and attains a regular rhythm and a higher rate as soon as the asystoles disappear at about 18oC (Eagles et al., 1988). This waxing and waning appearance of heart rate during arousal may be due to alternating sympathetic and parasympathetic dominance on the way to euthermia (Milsom et al., 1999). A ventricular bigeminy with a repetitive premature ventricular heart beats alternating with supraventricular beats is also demonstrated on ECG (Eagles et al., 1988). During mid to late arousal the heart rate, metabolism and respiratory frequency gradually reach a peak followed by body temperature under the influence of sympathetic tone until the animal reaches euthermia during which period the autonomic balance is restored (Lyman, 1958; Lyman and O’Brien, 1963; Twente and Twente, 1965; Lyman and O’Brien, 1969; Twente and Twente, 1978; Milsom et al., 1993).
Cardiac arrhythmias in hibernation vs hypothermia in hibernators
A study on ground squirrels revealed several differences in the ECG during hibernation and hypothermia (Dawe and Morrison, 1955; Nardone, 1955). A slow heart rate in hibernation is facilitated by a 40-70 fold increase in the duration of T-P segment suggesting a slowed SA node during hibernation. A 4-5 fold increase in duration of QRS complex is observed. About a 7 fold increase in the duration of P-R segment of an ECG indicates an increase in conduction time. On the other hand, a gradual decline in heart beats, appearance of right bundle branch block, and a notched QRS complex suggests a possibility of aberration in myocardial conduction. A reduced time span of QRS complex and a faster appearance of T wave soon after QRS complex were also noticed in hypothermia. Forced induction of hypothermia in Syrian hamsters induced J-waves and atrioventricular block while spontaneous hibernation had no adverse effect (Miyazawa et al., 2008). A study in ground squirrel has shown that decreased body temperature during spontaneous hibernation slows ventricular conduction velocity and increases excitation threshold thus avoiding arrhythmias at extreme low body temperatures (Fedorov et al., 2005).
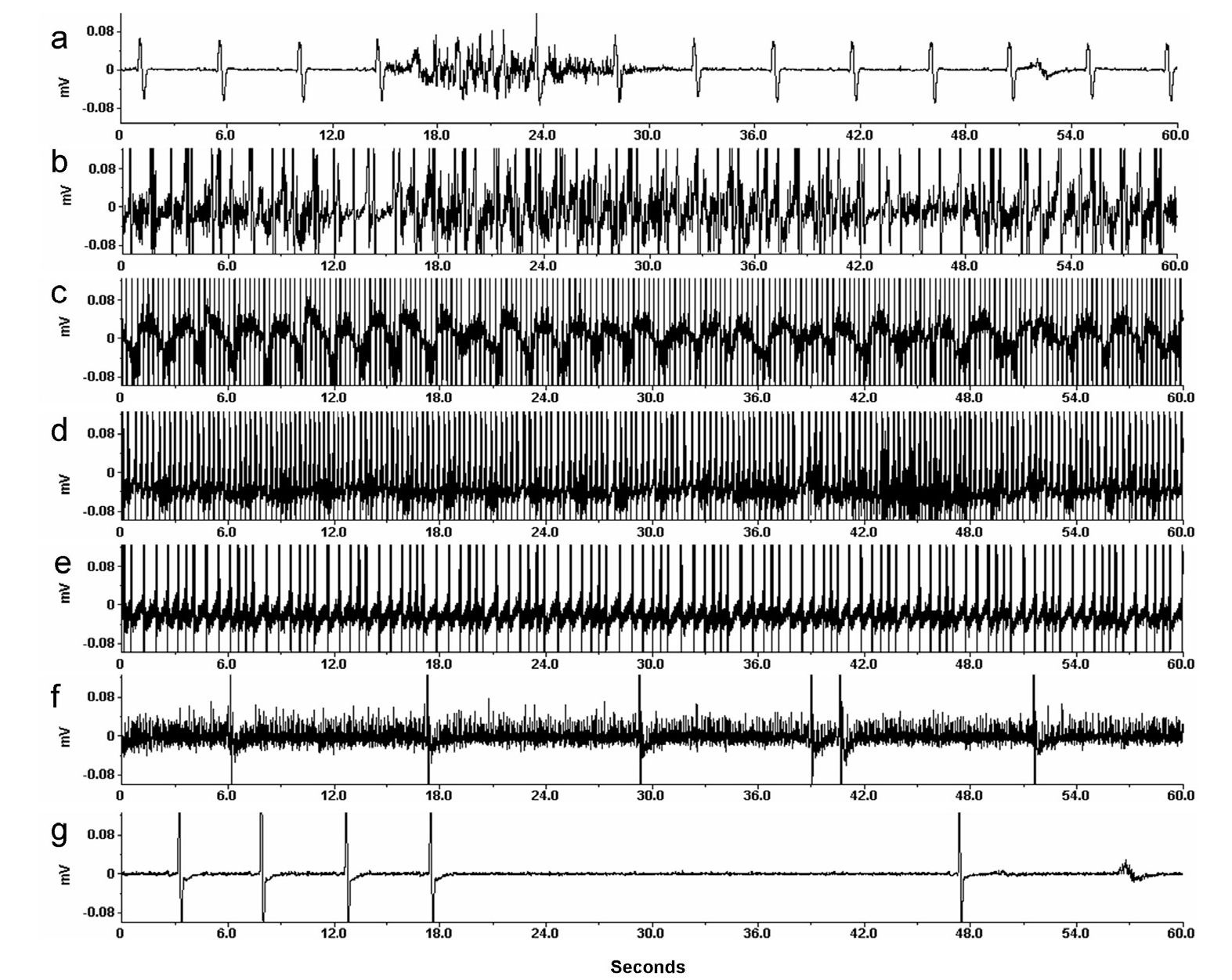
Fig. 3. Electrocardiogram of an arctic ground squirrel at different core body temperatures during different phases of hibernation.
Steady-state phase of hibernation is characterized by bradycardia with even beats as shown here at a body temperature of 4oC (a). An increase in heart rate occurs as soon as arousal is initiated as indicated in ECG at a body temperature of 5oC (b). A gradual progressive increase in heart rate is noticed through arousal phase at a body temperature of 20oC (c) until the animal reaches a euthermic body temperature of 37oC (d). Skipped beats and bradycardia follows as the animal prepares to enter into another torpor bout as represented by ECG at a body temperature of 36oC (e) and mid-entrance phase at 15oC (f). A brief pause in heart beats is a characteristic finding during the last stage of entrance phase (g) at a body temperature of 8oC.