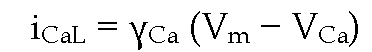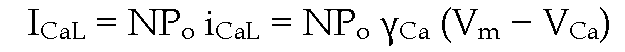Introduction
Cardiac arrhythmias result from the confluence of structural and functional changes in the heart and genetic predisposition, reflecting an interaction between a susceptible substrate (e.g. an anatomically defined circuit, a myocardial scar, fibrosis or a monogenic arrhythmia syndrome) and a specific electrophysiological triggering event. Such triggered activities arises from delayed afterdepolarizations (DADs) or early afterdepolarizations (EADs), in which action potential prolongation and aberrant Ca2+ fluxes are a recurrent theme. Ca2+ channels in cardiomyocytes provide the main influx pathway for Ca2+. Three types of high threshold Ca2+ channels are expressed in heart: two L-type channels, Cav1.2 and Cav1.3 and a P-type channel, Cav2.1. The Cav2.1 channel protein is expressed at a very low level the in heart (Starr et al., 1991) while Cav1.3 is mainly expressed in fetal hearts and only in adult sinoatrial and atrioventricular nodes and atrial tissues of adult (Lipscombe et al., 2004; Qu et al., 2005). We will focus attention on the Cav1.2 L-type Ca2+ channel (LTCC) which is the main player in electrical activity and excitation-contraction coupling (EC coupling) in the ventricular cardiomyocyte.
The LTCC of cardiomyocytes is a complex multimeric molecular sarcolemmal ensemble that during an action potential (AP) allows Ca2+ to flow down its electrochemical gradient into the cardiac cell. LTCCs are mostly localized in the transverse tubular system of cardiomyocytes (Wibo et al., 1991; Kawai et al., 1999; Brette et al., 2004). Activation of LTCC generates a Ca2+ current (ICaL) through the sarcolemma large enough to be involved in AP overshoot and in the control of AP duration (APD) in different cardiac cells types (Bers, 2001). ICaL serves as a trigger for Ca2+ release from the sarcoplasmic reticulum (SR) during the excitation-contraction coupling by a mechanism known as calcium-induced calcium release (CICR, Fabiato & Fabiato, 1975; Bers, 2001). LTCC activation can also play a role in transcription mechanisms in cardiomyocytes (Atar et al., 1995; Brette et al., 2006). Several hormones and neuromediators modulate the activity of LTCC via complex intracellular signaling pathways and, as well, several intracellular molecules and the cytoskeleton can influence LTCC activity (Benitah et al., 2010). However, intracellular Ca2+ concentration is strictly controlled in normal cells by different mechanisms (Bers, 2001) since a Ca2+ overload can have deleterious effects including arrhythmias and myocardial remodeling via a genetic reprogramming of the cardiac cell (Benitah et al., 2003).
Macromolecular structure
The typical structure of LTCC in ventricular cardiomyocytes is a macromolecular multimeric complex consisting of a ~240 kDa pore-forming unit a1C (encoded by the CACNA1C gene) and two auxiliary (modulator) subunits: an intracellular p subunit (mainly p2A, encoded by the CACNB2A gene) and the dimer a28 subunit (mainly a28-1, encoded by the CACNA2D1 gene) in a 1:1:1 ratio (Catterall et al., 2005). The a1C subunit consists of four homologous repeating motifs (I-IV), each one composed of six membrane-spanning a-helices (S1 to S6) linked by variable extracellular and cytoplasmic loops (linkers). This subunit contains all the necessary structures to allow the channel to gate (activation and inactivation) and confers the Ca2+ selectivity as well as the electrophysiological and pharmacological properties of the LTCC (Takahashi & Catterall, 1987; Catterall, 2000; Carafoli et al., 2001; Lacinova & Hoffmann, 2001; Bodi et al., 2005; Lacinova & Hofmann, 2005; Brette et al., 2006; Benitah et al., 2010). However, more native LTCC properties can be achieved when all three subunits are present (Lacinova & Hoffmann, 2001; Lacinova & Hofmann, 2005; Benitah et al., 2010). The p2A subunit seems to be involved in membrane targeting of a1C and influence LTCC inactivation (Bodi et al., 2005; Lacinova & Hofmann, 2005; Brette et al., 2006). Its structure reveals a module of two interacting domains, a Src homology 3 (SH3) domain and a Guanylate Kinase (GK) domain (Chen et al., 2004; Bodi et al., 2005). It was initially believed that its conserved sequence, BID (Beta Interaction Domain), interacted directly with the Alpha Interaction Domain (AID) within the intracellular loop between domains I and II of the a1C subunit (De Waard et al., 1996; Arikkath & Campbell, 2003). However, recent data indicate that BID is largely buried in the Cavp core and is unavailable for protein-protein interactions (Chen et al., 2004; Van Petegem et al., 2004). The AID is bound in a hydrophobic groove (a-binding pocket, ABP) in the GK domain and positions the p-subunit near the intracellular pore-lining segment I6 (which is important in Ca2+ channel inactivation) thus providing evidence that Cavp influence Ca2+ channel gating by direct modulation of this segment (Van Petegem et al., 2004). Although the BID does not participate directly in binding the a1C subunit, structural integrity and bridging of the SH3 and GK domains are greatly influenced by BID. The a28-1 subunit seems to be involved in targeting (or stabilization) of the a1C to the sarcolemma (Lacinova & Hoffmann, 2001; Lacinova & Hofmann, 2005; Brette et al., 2006; Benitah et al., 2010) and could confer more native LTCC properties. The 8 subunit is composed of a single transmembrane segment with a very short intracellular C-term and links by disulphide bonds the a2 subunit that is entirely extracellular (Davies et al., 2007). The a2 subunit contains a Von Willebrand factor A domain (VWA) that has a metal-ion-dependent adhesion site that seems to be key in trafficking the a1C subunit to the membrane (Canti et al., 2005).
Biophysics of the cardiac L-type Ca2+ channel
Selectivity and permeation
The cardiac LTCC is a multi-ion pore in which a Ca2+ ion bound to a high affinity site can be repelled by a second Ca2+ ion entering the pore thus allowing selectivity with high ionic flux (Hess & Tsien, 1984). The LTCC pore exhibits two different affinities for![]() for Ca2+ block of monovalent current through the channel and a
for Ca2+ block of monovalent current through the channel and a![]() for saturation of divalent current (which can be lower if surface charge screening is taken into account at high divalent concentrations), suggesting the existence of two Ca2+ binding sites within the channel’s pore (Almers & McCleskey, 1984; Hess & Tsien, 1984). In the absence of Ca2+ other ions can pass the channel and unitary conductance measurements gave the following sequence
for saturation of divalent current (which can be lower if surface charge screening is taken into account at high divalent concentrations), suggesting the existence of two Ca2+ binding sites within the channel’s pore (Almers & McCleskey, 1984; Hess & Tsien, 1984). In the absence of Ca2+ other ions can pass the channel and unitary conductance measurements gave the following sequence![]() (Hess et al., 1986). Four glutamate residues, one in each of the four P-loops of the LTCC (the EEEE locus), are important for channel selectivity (Tang et al., 1993; Yang et al., 1993). The current view of the selectivity filter considers that the EEEE locus is physically flexible (Sather & McCleskey, 2003). Some recent results suggest that an EEEE locus is not enough to explain selectivity and permeation in LTCC and other high voltage activated Ca2+ channels. A set of non conserved (channel specific) charged residues (Divalent Cation Selection or DCS locus) located in the upper half of the channel (pointing toward the pore) could form a second Ca2+ binding site important in defining a Ca2+ permeability profile. It was proposed that the number of charged residues in the DCS locus is critical for Ca2+ binding. In the cardiac LTCC the DCS locus contains three negative charges (DSED) that seem to be important for the high Ba2+ conductance (Cens et al., 2007).
(Hess et al., 1986). Four glutamate residues, one in each of the four P-loops of the LTCC (the EEEE locus), are important for channel selectivity (Tang et al., 1993; Yang et al., 1993). The current view of the selectivity filter considers that the EEEE locus is physically flexible (Sather & McCleskey, 2003). Some recent results suggest that an EEEE locus is not enough to explain selectivity and permeation in LTCC and other high voltage activated Ca2+ channels. A set of non conserved (channel specific) charged residues (Divalent Cation Selection or DCS locus) located in the upper half of the channel (pointing toward the pore) could form a second Ca2+ binding site important in defining a Ca2+ permeability profile. It was proposed that the number of charged residues in the DCS locus is critical for Ca2+ binding. In the cardiac LTCC the DCS locus contains three negative charges (DSED) that seem to be important for the high Ba2+ conductance (Cens et al., 2007).
Activation, inactivation and reactivation of LTCC
In a ventricular cardiomyocyte at rest (resting potential ~ -80 mV) there is a transmembrane Ca2+ concentration gradient (~ 2 mM outside, ~ 100 nM inside) that generates a huge driving force (electrochemical gradient) for Ca2+ that tends to move it into the cell. Activation of LTCC allows Ca2+ to enter the cardiomyocyte during the AP and constitutes the major Ca2+ entry pathway. With a threshold at -40 mV (or slightly positive to), activation of ICaL is fast with a time constant of 2-3 ms and time-to peak inward current ranging around 4-5 ms or less at the membrane potentials at which maximal inward current occurs (0 to +10 mV), and even faster at higher depolarizations (McDonald et al., 1994; Bers, 2001). Similar to Na+ channels, LTCC inactivate but with a much slower inactivation time course. With Ca2+ as charge carrier, ICaL inactivation is usually a biexponential process with an "U-shaped" voltage-dependence. Minimal values for time constants of 4 to 10 ms (xfast) and 40 to 60 ms (Tslow) occur at around 0 and +10 mV depending on cardiomyocyte type (McDonald et al., 1994; Bers, 2001). Deactivation of peak ICaL after a short depolarizing pulse and repolarization to a negative holding potential is fast with a time constant ranging between 0.2 and 0.5 ms (Josephson et al., 1984; Cohen et al., 1992). However, it can be slower in rat cardiomyocytes (~1 ms) (Richard et al., 1993).
Current-to-Voltage relationship
Current-to-voltage relationship for ICaL is bell-shaped with a threshold around -40 or -30 mV and a peak inward current at 0 (or +10 mV); it is almost linear at positive potentials and reverses around +60 to +70 mV at normal Ca2+ concentrations. At potentials beyond its reversal, ICaL exhibits some inward going rectification (McDonald et al., 1994; Bers, 2001). Whole cell ICaL can be roughly described by a Hodgkin-Huxley formalism considering that
where GCaL is the maximal Ca2+ conductance, d„ is the activation gate variable, f„ the inactivation gate variable, Vm is the membrane potential and VCa is the Ca2+ reversal potential (Luo & Rudy, 1994). Since ICaL inactivation is both voltage- and Ca2+-dependent (see below), the formalism can be more complex and could include a variable related to the Ca2+-dependent inactivation (CDI) process (Hirano & Hiraoka, 2003; Findlay et al., 2008). However, since the Hodgkin-Huxley formalism does not represent kinetic states of the ion channel, single channel-based Markov models could be more useful to fully describe coupling between kinetic gating transitions and molecular interactions in LTCC (Faber et al., 2007). At the single channel level, current-to-voltage relationship for LTCC is essentially ohmic over the whole potential range with some inward rectification near the reversal potential (McDonald et al., 1994). Since the single channel current iCaL can be described as
where![]() is the unitary conductance, the relationship between whole cell
is the unitary conductance, the relationship between whole cell![]() is
is
where N is the total number of functional channels and Po the probability that a channel is open.
Unitary conductance of LTCC is 3-5 pS when Ca2+ is the charge carrier and 15 – 25 pS with Ba2+ as charge carrier (McDonald et al., 1994; Bers, 2001; Guia et al., 2001). However, subconductance levels of 50% to 70% of the major conductance have been also demonstrated (McDonald et al., 1994). On depolarization LTCC activity can vary between different modes: gating mode 0 (or "null mode") in which the channel is not available to open; gating mode 1 (or "normal") consisting of short bursts of brief openings and closings and gating mode 2 (with high Po) in which the channel show long openings interrupted by short closings. This gating mode 2 is induced by phosphorylation, "Ca2+ channel agonists" (such as BAY K 8644) or strong depolarizations (Pietrobon & Hess, 1990; McDonald et al., 1994).
Voltage-dependence of activation and inactivation
Steady-state activation of IcaL (dro) has a sigmoidal relationship with the membrane potential with a half-activation potential around -15 mV. The relationship for the inactivation variable (fro; availability) is more complex since for potentials from -80 to 0 mV it is sigmoidal with a half-inactivation potential around -35 mV; however an "overshoot" can often be seen at potentials negative to -50 mV in cells clamped at negative holding potentials (> -80 mV). Other singularities of the availability curve of ICaL are that f„ rarely attains a zero value but a minimum between 0 and +10 mV and that the curve bends up at potentials positive to +10 mV, a phenomenon that is related to the CDI of ICaL (Mentrard et al., 1984). These characteristics d„ and f„ are consistently seen in cardiomyocytes from different species including humans (McDonald et al., 1994; Bers, 2001; Treinys & Jurevicius, 2008; Benitah et al., 2010).
Voltage- and Ca2+-dependent inactivation of LTCC
Time-dependent inactivation of ICaL during depolarization is both voltage- and Ca2+-dependent (Kass & Sanguinetti, 1984; Mentrard et al., 1984; Lee et al., 1985; Hadley & Hume, 1987). A very slow inactivation has also been described in the heart including human ventricular myocytes (Schouten & Morad, 1989; Benitah et al., 1992). CDI can be considered as the result of a two-component process, one due to Ca2+ ions passing through the channel and another due to Ca2+ release from the SR in the vicinity of the LTCC (Imredy & Yue, 1992; Richard et al., 2006; Faber et al., 2007). CDI can be easily shown up by using Ba2+ instead of Ca2+ as charge carrier which markedly prolonged LTCC inactivation time course. An increase in current amplitude is also seen since the LTCC has less affinity for Ba2+ than for Ca2+ (Hess et al., 1986). It is generally believed that under this condition, LTCC inactivation is essentially controlled by a voltage-dependent inactivation mechanism (VDI). However, this paradigm has been called into question since it has been well demonstrated that Ba2+ can induce ion (or current) -dependent inactivation (Markwardt & Nilius, 1988; Ferreira et al., 1997; Ferreira et al., 2003) and thus the "apparent VDI" with Ba2+ as charge carrier also shows fast and slow components. Evidences exist that VDI can also have fast and slow components (Hering et al., 2000; Ferreira et al., 2003; Findlay, 2004). The situation could be even more complicated since, at least for N-type Ca2+ channels, the permeant ion could interact in a complex way with the voltage sensor (Shirokov, 1999). The relative contribution of CDI to total inactivation of ICaL is still under dispute. It is commonly accepted that the fast inactivation phase of ICaL represents the CDI component (Findlay, 2004). However, it has been shown that the fast inactivation time constant of ICaL of rat ventricular cardiomyocytes was "unexpectedly" slowed down after ICaL was increased by P-adrenergic stimulation, as well as after manipulations not involving CDI (Alvarez et al., 2004; Haase et al., 2005; Alvarez et al., 2010). This makes difficult to ascertain which one of CDI or fast VDI predominates in the fast inactivation phase of ICaL with Ca2+ as charge carrier. Nevertheless, it has been suggested that VDI could be more important under control conditions and that after P-adrenergic stimulation, CDI becomes the main inactivation mechanism due to a slow down of VDI (Findlay, 2004). It should be noted that CDI could be visualized as a "Ca2+-dependent brake for a pre-existing voltage-dependent inactivation" based on the conserved regulation of both VDI and CDI by the auxiliary P-subunit, and that the I-II intracellular loop, essential for VDI, could also play a role in CDI (Cens et al., 1999; Cens et al., 2006). The precise mechanisms underlying CDI are not completely well defined. However, a general picture emerged in which in the presence of Ca2+ (entering through the LTCC or released from the SR) a calmodulin (CaM) molecule binds to the C-terminal tail of the a1C subunit to promote CDI. CaM binds to two segments (LA and IQ), in a Ca2+-dependent manner (Xiong et al., 2005) and it has been shown that the amino acid sequence of the IQ region in the a1C subunit is critical for CaM binding and CDI (Ohrtman et al., 2008). Several other structures seem to be involved in CDI such as an EF-hand locus in the C-terminus of a1C subunit (Peterson et al., 2000), the Cavp subunit (Zhang et al., 2005), the N-terminus of the a1C subunit, the I-II intracellular linker (Pitt et al., 2001; Erickson et al., 2003; Kobrinsky et al., 2005) and the pore region involved in slow inactivation (Shi & Soldatov, 2002).
Reactivation
Reactivation (removal of inactivation) of LTCC has been described as a mono or biexponential process, however, the time for half reactivation (t50) can be considered as a reliable parameter and has been reported to be in the range of 70-100 ms in cardiomyocytes clamped at negative holding potentials (-80 mV or more negative) and an overshoot at short coupling intervals is often seen. At more depolarized holding potentials, t50 can be notably increased and the overshoot disappears (Argibay et al., 1988; Tseng, 1988; Schouten & Morad, 1989; Alvarez & Vassort, 1992). A voltage-dependent transition into a closed available state and/or reopenings from the inactivated state could explain in part the reactivation of LTCC (Jones, 1991; Slesinger & Lansman, 1991). However, reactivation of LTCC is a more complex phenomenon since it is Ca2+-dependent (Argibay et al., 1988; Tseng, 1988) and thus related to CDI. The overshoot in ICaL reactivation could be of physiological relevance since it is, at least in part, related to the well-known increase in premature (extrasystolic) APD in well polarized cardiomyocytes but not in partially depolarized ones (Hiraoka & Sano, 1976).
Facilitation of LTCC
The overshoots seen in the availability and reactivation curves of ICaL are both a manifestation of a "facilitation" phenomenon of LTCC. In both cases an increase in Tfast is commonly observed and both seem to be related to the pacing-dependent (staircase) facilitation of ICaL (Lee, 1987). However, the "overshoots" and the staircase phenomena could be dependent on the basal ICaL density disappearing at higher current densities (Argibay et al., 1988; Alvarez & Vassort, 1992; Piot et al., 1996). Facilitation of LTCC has been more extensively studied by stimulating cardiomyocytes at high rates after a rest period or by applying prepulses of moderate and high amplitude (Richard et al., 2006). At negative holding potentials (> -80 mV) the frequency-dependent changes in ICaL amplitude upon stimulation can be variable: significant (Lee, 1987), modest or absent (Piot et al., 1996; Delgado et al., 1999) and even modestly decrease (Argibay et al., 1988; Alvarez & Vassort, 1992; Alvarez et al., 2004). However, an increase in Tfast has been consistently reported in these conditions, resulting, independently of what happens with ICaL amplitude, in a significant increase in Ca2+ influx (Delgado et al., 1999). The mechanism of this "Ca2+-dependent facilitation" (CDF) or potentiation seems to be related to a negative feedback involving less CDI at frequencies at which Ca2+ load and release from the SR are decreased (Delgado et al., 1999). This phenomenon has often been related to phosphorylation by cyclic AMP-dependent protein kinase (Tiaho et al., 1994; Piot et al., 1996) although there are also reports that p-adrenergic stimulation significantly diminished ICaL facilitation (Zygmunt & Maylie, 1990; Delgado et al., 1999; Alvarez et al., 2004). Disruption of the interaction between a1C and p2A subunits also abolished CDF (Alvarez et al., 2004). In this sense, this phenomenon is still far to be completely understood. CDF also involves CaM and the Ca2+/CaM kinase II (CaMKII). Similar to CDI, the CDF requires the binding of CaM to the IQ motif located in the a1C C-terminus but to a structural frame different to that involved in CDI (Zuhlke et al., 1999). Activation of CaMKII by Ca2+ entry or release from the SR is also involved in ICaL facilitation (Anderson et al., 1994; Yuan & Bers, 1994; Anderson, 2004). More recently phosphorylation of p2A has been reported to be critical for CaMKII-dependent ICaL facilitation (Grueter et al., 2006).
Similar to frequency-dependent facilitation, prepulse-induced facilitation of ICaL is characterized by an increase in Tfast (Barrere-Lemaire et al., 2000) and its underlying mechanism seems to involve a negative feedback on LTCC related to CDI as discussed above (Guo & Duff, 2003) and a positive feedback on LTCC following CaMKII activation by membrane potential and Ca2+ entry (Xiao et al., 1994).
LTCC "window" current
Activation and inactivation (availability) curves overlap at membrane potentials between the threshold for ICaL at -40 to potentials of 0 or +10 mV thus defining a "window" Ca2+ current (d„.f„ > 0) in the plateau range of the cardiac AP. The peak window current (which is proportional to d„.f„) is between -25 and -20 mV and could be as large as 10% of maximal ICaL (McDonald et al., 1994). Its existence has been verified in whole cell recordings (Hirano et al., 1992; McDonald et al., 1994). Within this window LTCC channels can cycle between closed, open and inactivated states but a transition again to the closed state and reopenings are possible before inactivating again (Shorofsky & January, 1992). Such reopenings have been clearly demonstrated in single channel recordings (Shorofsky & January, 1992; McDonald et al., 1994) and constitute the underlying mechanism for the EAD (January et al., 1988). EADs are more frequently observed at low rates when the APD is increased and during interventions that increase ICaL (e.g. after activation of P-adrenergic receptors). They are supposed to underlie the cellular mechanism of "Torsades de Pointes" (TdP) in long QT syndromes (Napolitano & Antzelevitch, 2011). Transient K+ outward current (Ito) reactivation at low rates could contribute to generation of EADs since it drives the membrane (plateau) potential to more negative "take off" potentials and warrants higher peak amplitude of EADs (January et al., 1988). P-adrenergic stimulation increases ICaL and shifts the window current to more negative potentials due to an increase in channel’s Po at more negative potentials (hyperpolarizing shifts in dc) and a shift of fco to more hyperpolarized potentials (McDonald et al., 1994) thus favoring the appearance of EADs. It is to be noted that, at these membrane potentials, the fast Na+ current (INa) and Ito are inactivated, the inward rectifier current IK1 is decreased and outward rectifier currents are just activating. As a result the total membrane resistance is increased (Weidmann, 1951) thus making the membrane space constant high enough to guarantee a rather high safety margin for the slow response to be conducted for a given ICaL density.
A note on "EADs" recorded in multicellular cardiac preparations
It is possible that in some cases EADs recorded in multicellular cardiac preparations represent a reentry from a distant site rather than a true EAD arising from the recording site. In any case, the mechanism underlying this activity is the same as the previously described for EAD. This reentry mechanism, at these short coupling intervals (during the AP plateau) is due to "slow response" APs that can be conducted with a large enough safety margin and are due to the activation of LTCC (Cranefield, 1975). The biophysical properties of LTCC described above, can fully account for the conducted slow response APs in partially depolarized cells. Under several pathological conditions these slow responses can be conducted and are at the origin of reentry, for example in depressed fibres in ischemia (Cranefield, 1975) since the slow response APs are rather resistant to hypoxia (Alvarez et al., 1981), in TdP associated to long QT syndromes (Antzelevitch & Burashnikov, 2001) (see below) or during the verapamil-sensitive reentrant intranodal tachycardia involving the AV node (Wellens et al., 1977).
A role for a second window current?
The characteristics of the activation and inactivation curves of ICaL could predict the existence of a "second window" current at potentials positive to +10 mV since at these values, the product dc.fc is > 0. Whether the overlap between dc (=1) and the increasing fc at positive prepulse potentials could represent a true "secondary" window current or not is debatable, but it is clear that after these prepulse potentials LTCCs recover from inactivation and reopen in a sort of "facilitation" (Pietrobon & Hess, 1990). Nonetheless, the physiological (or physiopathological) relevance of this window current is uncertain since at these membrane potentials the fast INa is the main depolarizing current and physiologically membrane potentials over +40 mV never exist. This property, however, has been important for the characterization of the CDI of LTCC in cardiac cells (Mentrard et al., 1984).