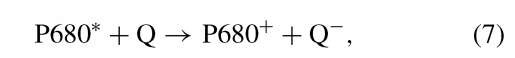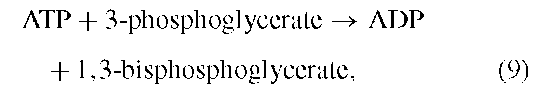From a purely thermodynamic standpoint, life is an improbable event. Consider, for example, the complex structures of organisms, not only at the macroscopic level, but also at the microscopic and atomic levels. These ordered structures can be formed and maintained only by the expenditure of energy. Within the ecosystem that we call the earth, the organic nutrients necessary to sustain the life of heterotrophs such as us are provided directly and indirectly by photosynthesis.
In both quantitative and qualitative terms photosynthesis is the most significant biological process on Earth. Approximately 2 x 1011 tons of carbon dioxide are converted to organic compounds each year. It is to photosynthesis in prehistoric times that we owe the reserves of fossil fuels. The oxygen that we breathe is a direct result of photosynthesis, now and in prehistory.
If the earth were an isolated system in a thermodynamic sense, life would be in jeopardy in that the energy reserves for life would be consumed. Without the input of energy from a source external to the earth, the planet must tend toward achieving equilibrium within its environment.
Fortunately, the earth is not an isolated system. The hydrogen fusion reactor of the Sun bathes our planet in electromagnetic radiation, including visible light. A fraction of the solar energy that impinges on Earth is converted by photosynthesis to chemical energy in the form of organic molecules that heterotrophic organisms use to satisfy their continued need for energy. The process by which light energy is used to drive the otherwise unfavorable synthesis of these organic molecules is called photosynthesis.
Although some bacteria carry out photosynthesis without the evolution of oxygen, this article deals solely with oxygenic photosynthesis that takes place in higher plants and algae. In a purely formal sense, oxygenic photosynthesis may be represented as the reverse of the oxidative breakdown of a six-carbon carbohydrate, such as glucose. An equation that describes photosynthesis in part illustrates this relationship:where C6H12O6 refers to a six-carbon sugar. This equation in reverse describes the oxidative catabolism of a six-carbon sugar such as glucose. Under standard conditions, the complete oxidation of glucose liberates 686 kcal/mol; the synthesis of a mole of glucose from carbon dioxide and water thus minimally requires the input of an equivalent amount of energy. In photosynthesis, visible light provides this energy. When it is considered that the only source of carbon for the tens of thousands of organic compounds synthesized in green plants is from the assimilation of carbon dioxide by means of photosynthesis, the inadequacy of Eq. (5) to describe photosynthesis, despite its usefulness, is readily apparent.
Inspection of Eq. (5) reveals that photosynthesis is an oxidation-reduction process. Simply put, photosynthesis is the light-driven reduction of carbon dioxide to the oxidation-reduction level of a carbohydrate by using water as the electron and hydrogen donor. In the process, water is oxidized to molecular oxygen. As stated previously, water is a very poor reducing agent. However, water at an effective concentration of 55 M is readily available in the biosphere. Although organic compounds and inorganic molecules such as hydrogen sulfide are more powerful reducing agents than water is, their use in photosynthesis as the source of electrons for photosynthesis is restricted to certain species of bacteria. The thermodynamically very unfavorable reduction of carbon dioxide by water is driven by light.
A. Light Reactions
How the electromagnetic energy of light is converted to chemical energy in the form of reduced organic molecules is complex. Nonetheless, the first principles of energy conservation and conversions in photosynthesis may be simply depicted. All higher photosynthetic organisms contain two forms of the green pigment chlorophyll. More than 99% of the chlorophyll in chloroplasts, the organelles in which photosynthesis takes place, functions in a passive, purely physical manner. Organized in specific pigment-protein complexes within the photosynthetic membrane, these chlorophylls absorb visible light and transfer excitation energy to nearby chlorophylls with efficiencies very close to 100%. In a real sense, more than 99% of the chlorophylls function only to gather light and as such they are often referred to as light-harvesting chlorophylls.
Within picoseconds of the harvesting, the excitation energy is transferred to specialized chlorophyll molecules called reaction center chlorophylls. These reaction center chlorophylls are identical to the majority of the light-harvesting chlorophylls. Yet, rather than acting in a passive manner when they are excited, the reaction center chlorophylls perform photochemistry. The two reaction center chlorophylls are termed P700 and P680. The "P" stands for pigment and the numbers refer to their absorption maxima, in nanometers, in the red region of the spectrum. The reaction center chlorophylls were first detected by light-induced bleaching at 680 and 700 nm. When the reaction center chlorophylls are excited, either directly or by resonance energy transfer from excited light-harvesting chlorophylls, an electron is transferred from the reaction center chlorophyll ensemble to an electron acceptor. These light-driven oxidation-reduction reactions occur within picoseconds and can operate with a quantum efficiency that is close to 100%. The reactions may be written as follows:
and
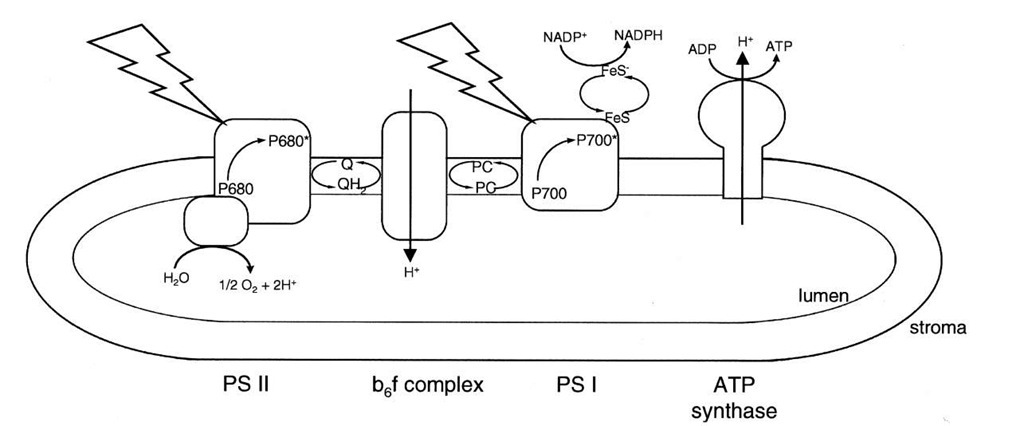
where the asterisks indicate the first excited singlet state of the reaction center chlorophyll, and FeS and Q are the redox active part of an iron-sulfur protein and a quinone, respectively, the first stable electron acceptors. P700+ and P680+ are chlorophyll cation radicals and Q— is a half reduced quinone and FeS- is a reduced iron-sulfur protein. The reactions shown in Eqs. (6) and (7) cannot take place, in the direction shown, in the dark when the reaction center chlorophylls are in the unexcited, ground state. The AG0 for both these reactions is approximately +24 kcal/mol. The excited reaction center chlorophylls are, however, much stronger reducing agents than the ground state chlorophylls are. The E0 of P700* is about 1.3 V more reducing than that of P700 in the ground state. These two electron transfer reactions are the only light-driven reactions in photosynthesis and they set the entire process in motion. The electron transport chain of chloroplasts is illustrated in Fig. 10.
Specific light-harvesting chlorophyll-protein complexes are associated with the reaction center chlorophyll-protein complexes in assemblies known as photosystems. Photosystem I (PS I) contains P700 and the FeS acceptor, and photosystem II (PS II), P680 and the quinone acceptor. Electron transfer in PS I generates a relatively weak oxidizing agent (P700+, E0 = +430 mV) and a strong reductant (FeS-, E0 = -600 mV). The primary reductant generated in photosynthesis is nicotinamide adenine din-ucleotide phosphate (NADP+), which, as the name suggests, differs from NAD+ by a single phosphate. While the physical properties of NADP+ and NAD+ are very similar, enzymes that use these pyridine nucleotides as substrates can discriminate between them by at least a factor of 1000. In general NAD+ is used in catabolic metabolism as we have seen for glycolysis and the tricarboxylic acid cycle. The reduced form of NADP+, NADPH, is, in contrast,used in biosynthesis, or anabolic metabolism. The E0 of the NADP+-NADPH redox pair is —340 mV. Thus, electron transfer from the reduced iron-sulfur protein of PS I to NADP+ is energetically a very favorable spontaneous reaction. It is NADPH that provides the electrons for CO2 reduction. The ultimate electron donor is water.
FIGURE 10 Electron transport and ATP synthesis in chloroplasts. The jagged arrows represent light striking the two photosystems (PS I and PS II) in the thylakoid membrane. Other members of the electron transport chain shown are a quinone (Q), the cytochrome complex (b6 f), plastocyanin (PC), and an iron-sulfur protein (FeS). The chloroplast ATP synthase is shown making ATP at the expense of the electrochemical proton gradient generated by electron transport.
Two water molecules are oxidized by PS II to yield four protons and molecular oxygen. Water is a very weak reducing agent. Thus, a strong oxidizing agent is needed for water oxidation. P680+ fits the bill. The midpoint potential of the P680+-P680 redox pair is on the order of + 1 V. Since the water-oxygen redox couple has an E0 of +0.815 V, the oxidation of water by P680+ is an energetically spontaneous reaction. Water oxidation is catalyzed by a manganese-containing enzyme that is plugged into the energy-converting thylakoid membrane.
So far, we have seen that the reduced FeS protein of PS I is converted to its oxidized form by passing electrons eventually to NADP+. In PS II, P680+ is reduced to P680 with electrons extracted from water. For electron transport to continue, the electron acceptor of PS II, Q—, and the electron donor of PS I, P700+, must be oxidized and reduced, respectively. The redox potential of the Q-Q— couple is about +0.05 V, whereas that of P700+-P700 is near +0.450 V. Thus, electron transport from Q— to P700+ is energetically spontaneous with a free energy of 9.3 kcal/mol for each electron transferred.
Electron transport from Q— to P700+ is mediated by a quinone, iron-sulfur, and a cytochrome protein complex in the thylakoid membrane. This protein, the cytochrome b6 f complex, is remarkably similar to the cytochrome bc1 complex of the mitochondrial electron transport chain.
B. CO2 Reduction
Linear electron transport in oxygenic photosynthesis is the reduction of NADP+ to NADPH by water, which results in the formation of molecular oxygen:
NADPH is incapable of reducing CO2 by itself; ATP is also required. The CO2 acceptor in photosynthesis is the five-carbon, phosphorylated sugar ribulose 1,5-bisphosphate. CO2 cleaves this sugar into 2 mol of the three-carbon sugar acid 3-phosphoglycerate, a compound that is also an intermediate in glycolysis. The enzyme that catalyzes this reaction, ribulose 1,5-bisphosphate carboxylase/oxygenase, or rubisco, is present in very high concentrations within chloroplasts, which makes it among the most abundant proteins in the biosphere.
Recall that in glycolysis one of the two steps in which ATP is formed is the conversion of 1,3-bisphosphoglycerate to 3-phosphoglycerate. The acylphosphate at the 1-position of the bisphosphorylated sugar acid is transferred to ADP to form ATP. The conversion of 3-phosphoglycerate to carbohydrates occurs by a pathway that is essentially the reverse of glycolysis. It must be emphasized, however, that glycolysis and photosynthetic carbon metabolism take place in separate intracellular compartments. Glycolysis occurs in the cytoplasm and uses NAD+ as the electron acceptor. The photosynthetic reduction of 3-phosphoglycerate occurs inside chloroplasts in the aqueous space known as the stroma. The enzymes in the two compartments are not the same even though they catalyze similar reactions. For example, the triose phosphate dehydrogenase in the cytoplasm is very specific for NAD+, whereas that in the chloroplast stroma is equally specific for NADPH.
Therefore, ATP is required for the reduction by NADPH of 3-phosphoglycerate to the oxidation level of a carbohydrate:
and the bisphosphoglycerate is in turn reduced by NADPH:
Since two 3-phosphoglycerates are generated for each CO2 assimilated, two NADPH and two ATP are required for reduction. This reaction is the only one in photo-synthetic carbohydrate metabolism that is an oxidation-reduction reaction.
Glyceraldehyde 3-phosphate is a sugar phosphate and may be readily converted within chloroplasts to many sugars and the glucose polymer starch. Some of the glyc-eraldehyde 3-phosphate is used in a complex series of reactions to regenerate the five-carbon acceptor of CO2, ribulose 1,5-bisphosphate. In the process, one phosphate is cleaved from one of the sugar phosphate intermediates. Thus, ribulose 5-phosphate, the product of the cycle, must be phosphorylated by using ATP as the phosphoryl donor. As a consequence, three ATP and two NADPH are required for each CO2 taken up.
Photosynthesis must satisfy the energy requirements of all living tissues in plants, including roots, stems, and developing fruit. Up to 75% of the triose phosphate formed is exported from the chloroplasts in leaf cells to the cytoplasm where it is converted to sucrose, a major product of photosynthesis. In most plants, sucrose is transported to the rest of the plant where it is either stored as starch or broken down by glycolysis and the citric acid cycle in exactly the same way as it is in animals to produce the ATP needed to sustain life.
C. ATP Synthesis
ATP synthesis in chloroplasts is called photophosphory-lation and is similar to oxidative phosphorylation in mitochondria. The light-driven transport of electrons from water to NADP+ is coupled to the translocation of protons from the stroma across the thylakoid membrane (the green, energy-converting membrane) into the lumen. Electron transport from Q— to P700+ is exergonic. Part of the energy released by electron transport is conserved by the formation of an electrochemical proton gradient. The cy-tochrome b6 f complex of chloroplasts functions not only in electron transport, but also in proton translocation.
The active site of the oxygen-evolving enzyme is arranged so that the protons formed during water oxidation are released into the thylakoid lumen. These protons contribute to the electrochemical proton potential. The thylakoid membrane contains aprotein that functions to transport Cl— across the membrane. Proton accumulation in the thylakoid lumen is electrically balanced in large part by Cl— uptake. As a result, thylakoids accumulate HCl and the membrane potential across the membrane is low. The pH inside the lumen during steady-state photosynthesis is about 5.0.
One of the earliest experiments that supported the hypothesis that ATP synthesis and electron transport were linked by the electrochemical proton potential was carried out with isolated thylakoid membranes. Thylakoid membranes were placed in a buffer at pH 4.0 and after a few seconds the pH was rapidly increased to 8.0, which resulted in the formation of a proton activity gradient. This artificially formed gradient was shown to drive the synthesis of ATP from ADP and Pi. The experiments were carried out in the dark so that the possibility that electron transport contributed to the ATP synthesis was excluded. Thus, a proton activity gradient was proven capable of driving ATP synthesis.
The thylakoid membrane enzyme that couples ATP synthesis to the flow of protons down their electrochemical gradient is called the chloroplast ATP synthase (see Fig. 10). This enzyme has remarkable similarities to ATP synthases in mitochondria and certain bacteria. For example, the fi subunits of the chloroplast ATP synthase have 76% amino acid sequence identity with the fi subunits of the ATP synthase of the bacterium E. coli.
The reaction catalyzed by ATP synthases is
where n is the number of protons translocated per ATP synthesized, probably three or four, and a and b refer to the opposite sides of the coupling membrane. Provided the electrochemical proton potential is high, the reaction is poised in the direction of ATP synthesis. In principle,when the proton potential is low, ATP synthases should hydrolyze ATP and cause the pumping of protons across the membrane in the direction opposite that which occurs during ATP synthesis. ATP-dependent proton transport by the ATP synthase is of physiological significance in E. coli under anaerobic conditions in that it generates the electrochemical proton potential across the plasma membrane of the bacterium. This potential is used for the active uptake of some carbohydrates and amino acids.
In contrast, ATP hydrolysis by the chloroplast ATP syn-thase in the dark has no physiological role and would be wasteful. In fact, the rate of ATP hydrolysis by the ATP synthase in thylakoids in the dark is less than 1% of the rate of ATP synthesis in the light. Remarkably, within 10-20 msec after the initiation of illumination, ATP synthesis reaches its steady-state rate. Thus, the activity of the chloroplast ATP synthase is switched on in the light and off in the dark. In addition to being the driving force for ATP synthesis, the electrochemical proton potential is involved in switching the enzyme on. Structural perturbations of the enzyme induced by the proton potential overcome inhibitory interactions with bound ADP as well as with a polypeptide subunit of the synthase. An additional regulatory mechanism that is unique to the chloro-plast ATP synthase is reductive activation. Reduction of a disulfide bond in a subunit of the chloroplast ATP synthase to a dithiol enhances the rate of ATP synthesis, especially at physiological values of the proton potential. The electrons for this reduction are derived from the chloroplast electron transport chain.