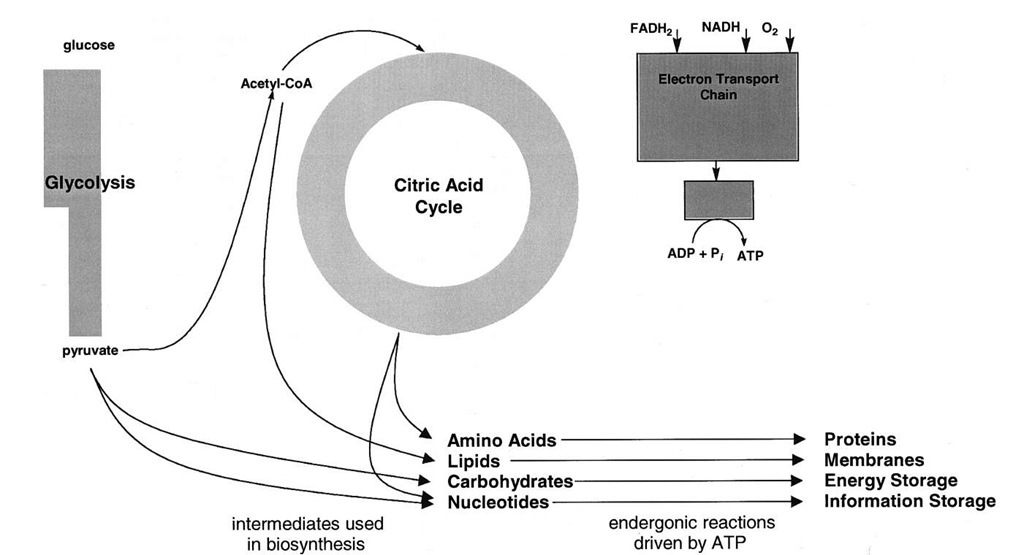
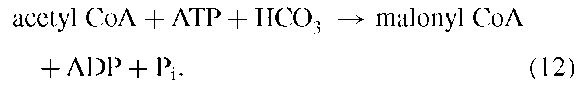ATP powers most of the endergonic processes in cells. How the potential energy of the phosphoanhydride bond of ATP may be used to drive otherwise unfavorable reactions (Fig. 11) is discussed in this section. This discussion focuses on three major uses of ATP: the generation of ion gradients, biosynthesis, and movement.
A. Ion Transport
The plasma membrane is the barrier that separates the cytoplasm of cells from the exterior medium. All cells maintain a membrane potential that is negative. There is an excess of positive charge in the external medium in comparison with that in the cytoplasm. The membrane potential in plant cells can be as high as -200 mV. Energy is required to generate and maintain the membrane potential.
All cells maintain gradients in ions across the plasma membrane. The intracellular K+ concentration is higher than that of the extracellular medium, and the concentration of Na+, much lower. The free Ca2+ concentration in the cytoplasm is maintained at very low levels, 1000fold or more below the extracellular Ca2+ concentration. Often the intracellular proton concentration can be quite different from that in the medium. The pH in the cytoplasm of plant cells is close to 7.0, whereas that in the medium is about 5.0. Energy is needed to generate and maintain these ionic disequilibria. For example, the energy cost to generate a pH gradient of two pH units is equal to RT ln([H+]/[H+]), where the subscripts o and i stand for outside and inside the cell, respectively. At 25°C, the AG’ for a 100-fold proton activity (pH 7.0 in versus pH 5.0 out) gradient is 2.7 kcal/mol.
Plasma membranes of all higher organisms contain enzymes that are embedded in the membrane that act as ion pumps. That is, they catalyze the transport of ions against their electrochemical potential. In physiology, transport that is thermodynamically uphill is termed active transport to distinguish it from the spontaneous flow of ions down their electrochemical potential. The energy needed for the active transport of ions across the plasma membrane is provided by the hydrolysis of ATP to ADP and Pi. As much as 75% of cellular ATP may be consumed simply to generate and maintain ion gradients.
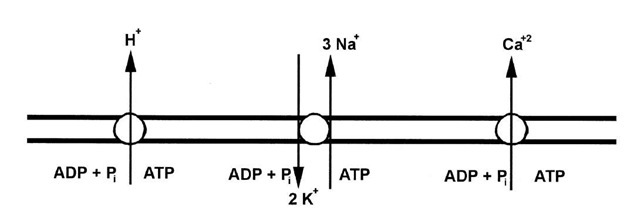
The electrogenic ion pump in the plasma membrane of animal cells is the Na+/K+-ATPase. As shown in Fig. 12, three Na+ ions are transported out of the cell and two K+ ions are pumped in for each ATP that is hydrolyzed. Since three positively charged ions are exported, but only two imported, the Na+/K+ -ATPase is electrogenic. The trans plasma membrane potential is on the order of —50 mV. In addition, the pump keeps the intracellular Na+ concentration nearly 100-fold lower than that in the serum, and the intracellular concentration of K+, about 30-fold higher than in serum.
FIGURE 11 Uses of ATP. The diagram shows some of the major processes in cells that are powered by ATP hydrolysis.
Indirectly, the Na+/K+ -ATPase provides the energy for the active transport of amino acids and some carbohydrates into cells. The plasma membrane contains specific proteins that mediate the transport of these molecules in a manner that is obligatorily linked to the cotransport of Na+. Since the extracellular Na+ concentration is higher than that in the cytoplasm and the membrane potential is negative, theNa+ flows from outside to inside the cell. Assuming a membrane potential of -50 mV and a 100-fold Na+ concentration gradient, the flow of Na+ would liberate about 3.8 kcal/mol at 25°C. This exergonic flow of Na+ provides the energy needed for the active transport of the amino acid or carbohydrate. Although Na+ flux is the immediate source of energy for the active transport in Na+-linked transporters, it is important to keep in mind that the ultimate energy source is ATP hydrolysis by the Na+/K+-ATPase.
FIGURE 12 Some ion pumps in the plasma membrane. The Na+/K+ -ATPase of animal cells uses the energy of ATP hydrolysis to move three Na+ ions out of the cells and two K+ ions in, which results in the generation of ion gradients and a membrane potential. Plant, yeast, and fungal cells do not have a Na+/K+ -ATPase, but instead have a H+-ATPase, as the electrogenic pump. The plasma membrane also contains a Ca2+-ATPase that pumps Ca2+ out of cells to help keep the intracellular Ca2+ concentration low.
Plants, yeasts, and fungi do not contain a Na+/K+-ATPase in their plasma membranes. Instead, they contain aH+-ATPase that is the generator of the plasma membrane potential. The H+-ATPase is structurally and mechanistically related to the Na+/K+ -ATPase but translocates only H+. The H+-ATPase is capable of generating large electrochemical proton gradients. The imbalance in the Na+ and K+ concentrations between the inside and the outside of the plant cell is maintained by other mechanisms that include exchange transport of Na+ for H+.
The active transport of some organic molecules across the plasma membrane of plants, yeasts, and fungi is linked to the cotransport of H+ down its eletrochemical gradient into the cell. An important example of proton-linked transport is that of sucrose loading into the vascular element, the phloem, that transports sucrose from the leaves to the remainder of a plant. The concentration of sucrose in phloem cells near leaves that are actively carrying out photosynthesis can be 0.5 M or higher, whereas that in the intracellular space, just 0.001 M. The energy cost of generating this gradient is 3.7 kcal/mol at 25°C. The immediate source of energy is proton flow, and the ultimate source, ATP hydrolysis by the H+-ATPase.
The concentration of free Ca2+ (meaning that unbound to proteins and membrane lipids) in the cytoplasm of cells is normally maintained at a very low level. Under certain circumstances, however, transient increases in the cytoplasmic Ca2+ concentration are triggered. Ca2+ is a major player in the transmission of some hormonally induced signals in plants and animals. Muscle contraction is also induced by release of Ca2+ from internal membranes within muscle cells.
The plasma membrane contains an enzyme that catalyzes the export of Ca2+ from the cytoplasm at the expense of ATP hydrolysis. The Ca2+-ATPase has features that place it in the category of plasma membrane enzymes that also includes the Na+/K+-ATPase and the H+-ATPase. The Ca2+-ATPase functions to keep the cy-tosolic Ca2+concentration low (< 1 \xM). It is not a major contributor to the generation of the membrane potential or to the energetics of the transport of bioorganic molecules.
Inhibitors of the enzyme responsible for the acidification of the stomach are well known and equally well-advertised alleviators of "heartburn." This enzyme is present in the parietal cells of the stomach and resembles the Na+/K+-ATPase. Instead of catalyzing the ATP-dependent exchange of Na+ and K+, the stomach acid pump excretes H+ into the lumen of the stomach in exchange for K+.
B. Biosynthetic Use of ATP
The input of energy in the form of the hydrolysis of ATP to either ADP and Pi or to adenosine monophosphate (AMP) and pyrophosphate powers the synthesis ofbiolog-ical molecules, including, as we have seen, carbohydrates in photosynthesis, proteins, DNA, RNA, and fatty acids. To delve into the role of ATP in biosynthesis in depth is not possible in this brief article. Aspects of fatty acid biosynthesis, however, reveal interesting principles of the energetics of biosynthetic pathways.
Fatty acids are oxidized completely to CO2 and water by j-oxidation and the citric acid cycle. Acetyl CoA is the end product of j-oxidation of fatty acids and is the source of carbon for fatty acid biosynthesis. Yet, the pathways for fatty acid degradation and synthesis are so very different that they even occur within different compartments within cells. Fatty acid synthesis takes place in the cytoplasm of animal cells and in the plastids of plant cells, whereas j -oxidation is located in mitochondria in both animal and plant cells.
Often, the pathway for the synthesis of a compound differs significantly from that for its degradation. Among the reasons that the separation of synthetic and degrada-tive pathways evolved are energetics and regulation. The oxidation of fatty acids to acetyl CoA is very exergonic. It is not feasible on energetic grounds to make fatty acids from acetyl CoA by reversing j-oxidation. Metabolism of carbohydrates and fats is regulated in mammals by a number of hormones, including insulin, glucagon, and epinephrine (adrenaline). Having separate pathways for the degradation and the biosynthesis makes it possible to turn off one pathway while up-regulating another. For example, glucagon and epinephrine selectively stimulate the breakdown of fats and fatty acids, whereas insulin has the opposite effect. The fine control of fatty acid metabolism that has evolved would clearly not be possible without the existence of separate pathways for biosynthesis and catabolism.
CO2 is required for the synthesis of fatty acids. Yet, when fatty acid synthesis is carried out in the presence of radioactive CO2, the fatty acid made is devoid of radioactivity. ATP is used to add CO2 to a precursor, and in a subsequent step in the pathway of fatty acid biosynthesis,this same CO2 is released. This seemingly perplexing phenomenon may readily be explained on an energetic basis.
The enzyme that catalyzes this reaction, acetyl CoA car-boxylase, contains biotin, one of the B vitamins. Several other vitamins, including niacin (part of NAD+ and NADP+) and riboflavin (part of FAD), are essential players in metabolism.
The carboxylation of acetyl CoA without the hydrolysis of ATP is energetically unfavorable. The exergonic hydrolysis of ATP pulls the reaction toward malonyl CoA synthesis. But why bother to carboxylate acetyl CoA?
All the carbon atoms in synthesized fatty acids are derived from the acetyl group of acetyl CoA. In principle, fatty acids could be made by condensation of acetyl units and subsequent reduction. However, the condensation of two acetyl CoA molecules is energetically unfavorable. The release of CO2 as part of a reaction helps to drive a reaction to completion. The oxidative decarboxylation reactions of the citric acid cycle illustrate this fact. The loss of CO2 from the malonyl group as it condenses with the acetyl group bound to the fatty acid synthetase drives the condensation reaction. The resulting fi-keto compound is reduced to the level of a hydrocarbon by NADPH.
ATP hydrolysis provided the energy for the carboxy-lation of acetyl CoA. The immediate energy source for the condensation reaction was the loss of the same CO2 molecule added to the acetyl CoA. It is clear that CO2 plays a catalytic but essential role in fatty acid biosynthesis.
C. ATP and Motility
At macroscopic and microscopic levels, ATP hydrolysis results in movements. The most familiar of these movements are those caused by muscle contraction. Muscle contraction is an example of the conversion of the phosphate bond (chemical) energy of ATP to mechanical energy. Vertebrate muscle is composed of two types of filaments, thick and thin. The protein myosin is the major component of the thick filaments, whereas actin and other proteins make up the thin filaments. The thick and the thin filaments are interdigitated. Muscle contraction is thought to take place by a sliding of the thin filaments relative to the thick filaments. Myosin has ATPase activity. The catalytic site in myosin is located on a part of the molecule (the head) that interacts with the actin filaments. ATP hydrolysis is thought to cause changes in the interactions of the myosin head with the actin filaments such that the head moves along the actin filament in one direction.
Acetyl CoA is carboxylated by using bicarbonate as the source of CO2 and ATP hydrolysis as the source of energy:
Muscle contraction is regulated by a Ca2+-binding protein in the thin filaments. Ca2+ is required for muscle contraction. During rest, the concentration of Ca2+ in muscle cells is kept low by the operation of two Ca2+-ATPases, one in the plasma membrane and the other in internal membranes called the sarcoplasmic reticulum. The release of Ca2+ triggers muscle contraction, and its uptake into the lumen of the sarcoplasmic reticulum causes relaxation.