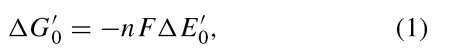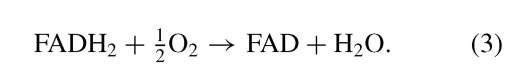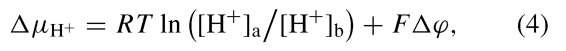Metabolism may be defined as the total of all the chemical reactions that occur in organisms. Green plants can synthesize all the thousands of compounds they contain from carbon dioxide, water, and inorganic nutrients. The discussion of the complicated topic of metabolism is somewhat simplified by separation of the subject into two areas—catabolic and anabolic metabolism. Catabolic metabolism is degradative and is generally exergonic. ATP is a product of catabolic metabolism. In contrast, anabolic metabolism is synthetic and requires ATP. Fortunately, there are relatively few major pathways of energy metabolism.
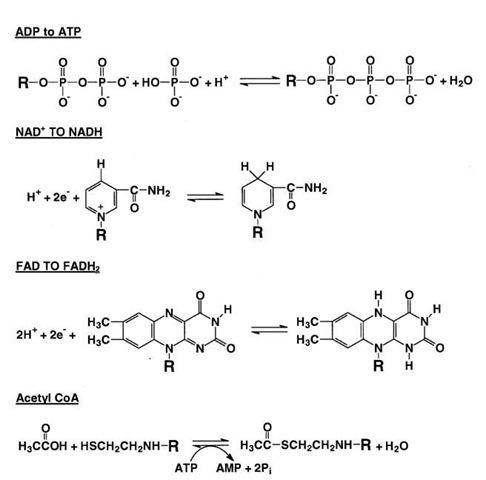
FIGURE 2 Some important reactions in metabolism. Shown are the phosphorylation of ADP to ATP, NAD+, NADH, FAD, FADH2 acetate, CoA, and acetyl CoA. For clarity, just the parts of the larger molecules that undergo reaction are shown. NAD+, nicotinamide adenine dinucleotide; NADH, nicotinamide adenine dinu-cleotide (reduced form); FAD, flavin adenine dinucleotide; FADH2, flavin adenine dinucleotide (reduced form); CoA, coenzyme A; AMP, adenosine monophosphate.
A. Glycolysis and Fermentation
Carbohydrates are a major source of energy for organisms. The major pathway by which carbohydrates are degraded is called glycolysis. Starch, glycogen, and other carbohydrates are converted to the sugar glucose by pathways that will not be considered here. In glycolysis, glucose, a six-carbon sugar, is oxidized and cleaved by enzymes in the cytoplasm of cells to form two molecules of pyruvate, a three-carbon compound (see Figs. 3 and 4). The overall reaction is exergonic and some of the energy released is conserved by coupling the synthesis of ATP to glycolysis.
Before it may be metabolized, glucose must first be phosphorylated on the hydroxyl residue at position 6. Under intracellular conditions, the direct phosphorylation of glucose by Pi is an unfavorable reaction, characterized by a AG0 of about 4 kcal/mol, at pH 7.0 and 25°C. (Note that the biochemist’s standard state differs from that as usually defined in that the activity of the hydrogen ion is taken as 10-7 M, or pH 7.0, rather than 1 M, or pH 0.0. pH 7.0 is much closer to the pH in most cells.) This problem is neatly solved in cells by using ATP, rather than Pi, as the phosphoryl donor:
The AG0 for this reaction, which is catalyzed by the enzyme hexokinase, is approximately —4 kcal/mol. Thus the phosphorylation of glucose by ATP is an energetically favorable reaction and is one example of how the chemical energy of ATP may be used to drive otherwise unfavorable reactions.
Glucose 6-phosphate is then isomerized to form fructose 6-phosphate, which in turn is phosphorylated by ATP at the 1-position to form fructose 1,6-bisphosphate. It seems odd that a metabolic pathway invests 2 mol of ATP in the initial steps of the pathway when ATP is an important product of the pathway. However, this investment pays off in later steps.
Fructose 1,6-bisphosphate is cleaved to form two triose phosphates that are readily interconvertible. Note that the oxidation-reduction state of the triose phosphates is the same as that of glucose 6-phosphate and the fructose phosphates. All molecules are phosphorylated sugars. In the next step of glycolysis, glyceraldehyde 3-phosphate is oxidized and phosphorylated to form a sugar acid that contains a phosphoryl group at positions 1 and 3. The oxidizing agent, nicotinamide adenine dinucleotide (NAD+), is a weak oxidant (E0, at pH 7.0 of —340 mV). The oxidation of the aldehyde group of glyceraldehyde 3-phosphate to a carboxylate is a favorable reaction that drives both the oxidation and the phosphorylation. This is the only oxidation-reduction reaction in glycolysis.
The hydrolysis of acyl phosphates, such as that of position 1 of 1,3-bisphosphoglycerate, is characterized by strongly negative AG0 values. That for 1,3-bisphos-phoglycerate is approximately —10 kcal/mol, which is significantly more negative than the AG0 for the hydrolysis of ATP to ADP and Pi. Thus, the transfer of the acyl phosphate from 1,3-bisphosphoglycerate to ADP to form 3-phosphoglycerate and ATP is a spontaneous reaction. Since two sugar acid bisphosphates are formed per glucose metabolized, the two ATP invested in the beginning of the pathway have been recovered.
In the next steps of glycolysis, the phosphate on the 3-position of the 3-phosphoglycerate is transferred to the hydroxyl residue at position 2. Removal of the elements of water from 2-phosphoglycerate results in the formation of an enolic phosphate compound, phospho(enol)pyruvate (PEP). The free energy of hydrolysis of PEP to form the enol form of pyruvate and Pi is on the order of —4 kcal/mol. In aqueous solution, however, the enol form of pyruvate is very unstable. Thus, the hydrolysis of PEP to form pyruvate is a very exergonic reaction. The AG0 for this reaction is —14.7 kcal/mol, which corresponds to an equilibrium constant of 6.4 x 1010. PEP is thus an excellent phosphoryl donor and the formation of pyruvate is coupled to ATP synthesis. Since two molecules of pyruvate are formed per glucose catabolized, two ATP are formed. Thus the net yield of ATP is two per glucose oxidized to pyruvate.
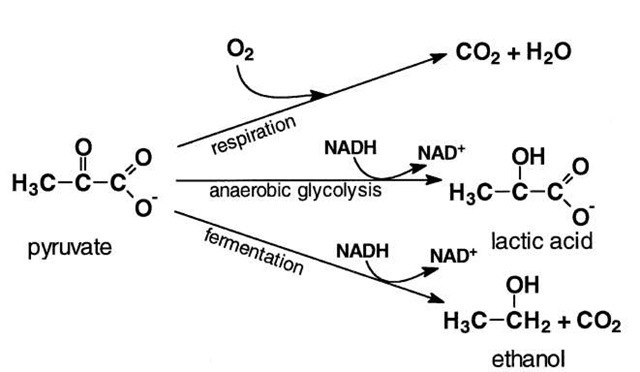
In some organisms, glycolysis is the only source of ATP. A familiar example is yeast growing under anaerobic (no oxygen) conditions. In this case, glucose is said to be fermented and ethyl alcohol and carbon dioxide (CO2) are the end products (Fig. 5). In contrast, all higher organisms can completely oxidize pyruvate to CO2 and water, using molecular oxygen as the terminal electron acceptor. The conversion of glucose to pyruvate releases only a small fraction of the energy available in the complete oxidation of glucose. In aerobic organisms, more than 90% of the ATP made during glucose catabolism results from the oxidation of pyruvate.
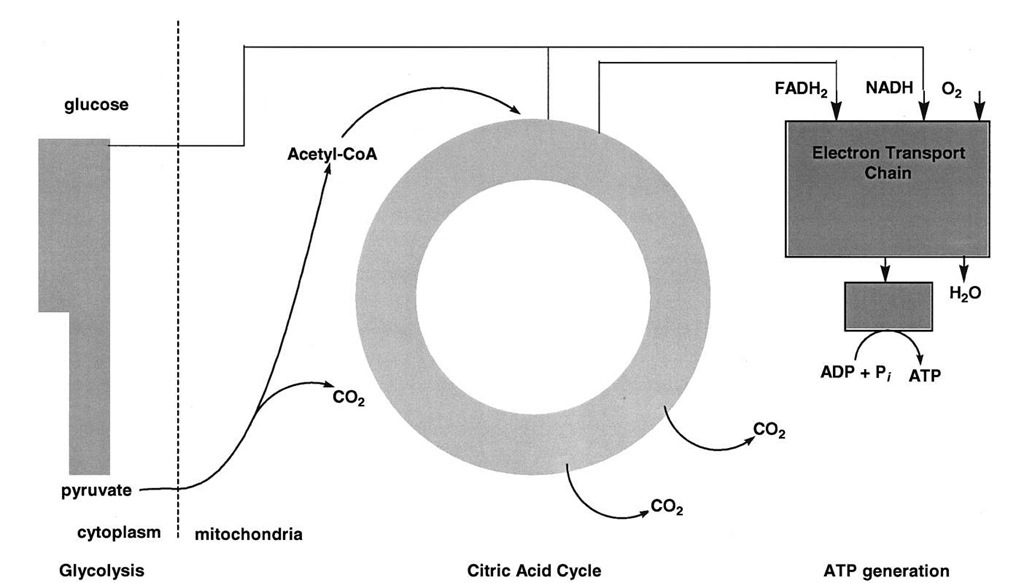
FIGURE 3 Schematic outline of carbohydrate metabolism. Glucose is oxidized to two molecules of pyruvate by glycolysis in the cytoplasm. In mitochondria, pyruvate is oxidized by molecular oxygen to CO2 and water. The synthesis of ATP is coupled to pyruvate oxidation.
B. Oxidation of Pyruvate: The Citric Acid Cycle
In higher organisms, the oxidation of pyruvate takes place in subcellular, membranous organelles known as mitochondria. Because mitochondria are responsible for the synthesis of most of the ATP in nonphotosynthetic tissue, they are often referred to as the powerhouses of cells. Mitochondrial ATP synthesis is called oxidative phos-phorylation since it is linked indirectly to oxidative reactions. In the complete oxidation of pyruvate, there are five oxidation-reduction reactions. Three of these reactions are oxidative decarboxylations. The electron acceptor (oxidizing agent) for four of the reactions is NAD+; the oxidizing agent for the fifth is flavin adenine dinucleotide, or FAD.
Knowing the oxidation-reduction potentials of the reac-tants in an oxidation-reduction reaction permits the ready calculation of the standard free energy change for the reaction. It may be shown that
where n is the number of electrons transferred in the reaction, F is Faraday’s constant (23,060 cal/V-equivalent), and A E0 is the difference between the E0 value of the oxidizing agent and that of the reducing agent.
The reduced form of NAD+, NADH, is a strong reducing agent. The E0 at pH 7.0 of the NAD+-NADH couple is —340 mV, which is equivalent to that of molecular hydrogen. E0 is the potential when the concentrations of the oxidized and reduced species of an oxidation-reduction pair are equal. Reduced FAD, FADH2, is a weaker re-ductant than NADH, with an E0 (pH 7.0) of about 0 V. In contrast, molecular oxygen is a potent oxidizing agent and fully reduced oxygen, water, is a very poor reducing agent. The E0 (pH 7.0) for the oxygen-water couple is +815 mV.
FIGURE 4 A view of glycolysis. Glucose, a six-carbon sugar, is cleaved and oxidized to two molecules of pyruvate. There is the net synthesis of two ATP per glucose oxidized and two NADH are also formed.
The oxidation of NADH and FADH2 results in the reduction of oxygen to water:
and
In both cases two electrons are transferred to oxygen, so that the n in Eq. (1) is equal to 2. Under standard conditions, the oxidation of 1 mol of NADH by oxygen liberates close to 53 kcal, whereas the AG0 for that of FADH2 is —38 kcal/mol. These two strongly exergonic reactions provide the energy for the endergonic synthesis of ATP.
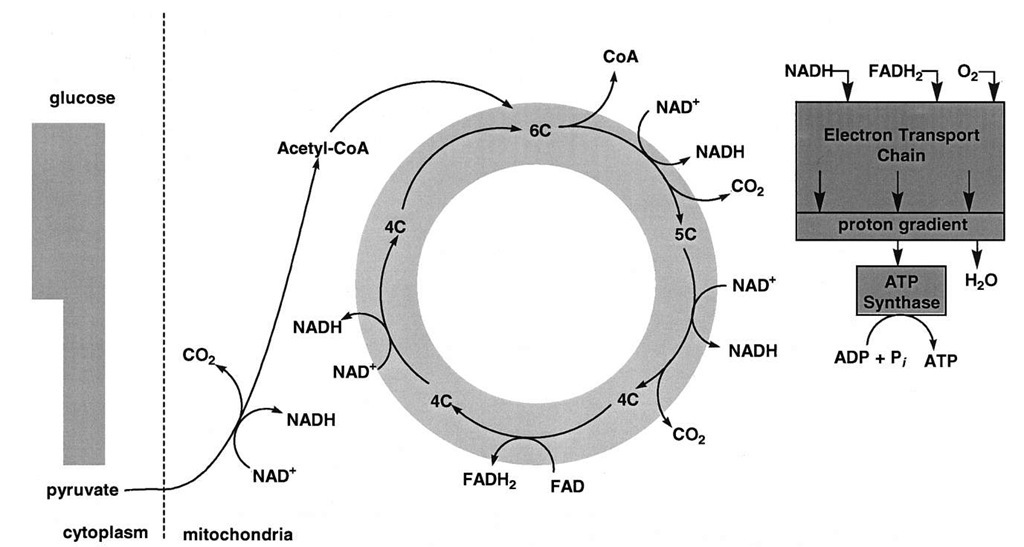
The details of carbon metabolism in the citric acid cycle are beyond the scope of this article. In brief, pyruvate is first oxidatively decarboxylated to yield CO2, NADH, and an acetyl group attached in an ester linkage to a thiol on a large molecule, known as coenzyme A, or CoA. (See Fig. 2.) Acetyl CoA condenses with a four-carbon dicar-boxylic acid to form the tricarboxylic acid citrate. Free CoA is also a product (Fig. 6). A total of four oxidation-reduction reactions, two of which are oxidative decarboxylations, take place, which results in the generation of the three remaining NADH molecules and one molecule of FADH2. The citric acid cycle is a true cycle. For each two-carbon acetyl moiety oxidized in the cycle, two CO2 molecules are produced and the four-carbon dicarboxylic acid with which acetyl CoA condenses is regenerated.
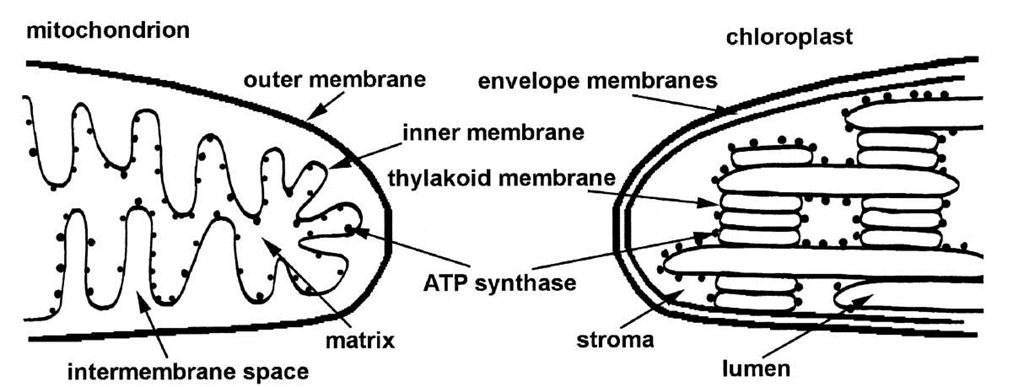
The mitochondrial inner membrane (Fig. 7) contains proteins that act in concert to catalyze NADH and FADH2 oxidation by molecular oxygen. [See reactions (2) and (3) above.] These reactions are carried out in many small steps by proteins that are integral to the membrane and that undergo oxidation-reduction. These proteins make up what is called the mitochondrial electron transport chain. Components of the chain include iron proteins (cytochromes and iron-sulfur proteins), flavoproteins (proteins that contain flavin), copper, and quinone binding proteins.
The oxidation of NADH and FADH2 by molecular oxygen is coupled in mitochondria to the endergonic synthesis of ATP from ADP and Pi. For many years the nature of the common intermediate between electron transport and ATP synthesis was elusive. Peter Mitchell, who received a Nobel Prize in chemistry in 1978 for his extraordinary insights, suggested that this common intermediate was the proton electrochemical potential. He proposed in the early 1960s that electron transport through the mitochondrial chain is obligatorily linked to the movement of protons across the inner membrane of the mitochondrion. In this way, part of the energy liberated by oxidative electron transfer is conserved in the form of the proton electrochemical potential. This potential, A^H+, is the sum of contributions from the activity gradient and that of the electrical gradient:
where R is the gas constant; T, the absolute temperature; a and b, the aqueous spaces bounded by the membrane; F, Faraday’s constant; and Ay, the membrane potential. As Mitchell suggested, the mitochondrial inner membrane is poorly permeated by charged molecules, including protons. The membrane thus provides an insulating layer between the two aqueous phases it separates. Thus the transport of protons across the membrane generates an electrochemical potential. In the case of mitochondria, the membrane potential is the predominant component of the electrochemical of the proton. The total A^H+ in actively respiring mitochondria is on the order of —200 mV, if one uses the convention that the inside space bounded by the membrane is negative.
FIGURE 5 Fates of pyruvate. In yeasts under anaerobic conditions, pyruvate is decarboxylated and reduced by the NADH formed by glycolysis to ethanol. In anaerobic muscle, the NADH generated by glycolysis reduces pyruvate to lactic acid. When O2 is present, pyruvate is completely oxidized to CO2 and water.
Electron transport from NADH and FADH2 to oxygen provides the energy for the generation of the electrochemical potential of the proton. The flow of protons down this potential is exergonic and is the immediate source of energy for ATP synthesis. The proton-linked synthesis of ATP is catalyzed by a complex enzyme called ATP syn-thase. Remarkably similar enzymes are located in the coupling membranes of bacteria, mitochondria, and chloro-plasts, the intracellular sites of photosynthesis in higher plants. Even though the reaction that they catalyze seems relatively straightforward (see Fig. 2), the ATP synthases contain a minimum of 8 different proteins and a total of about 20 polypeptide chains.
FIGURE 6 A view of the oxidation of pyruvate. The oxidation of pyruvate generates three CO2, four NADH, and one FADH2. The oxidation of NADH and FADH2 by the mitochondrial electron transport chain is exergonic and provides most of the energy for ATP synthesis.
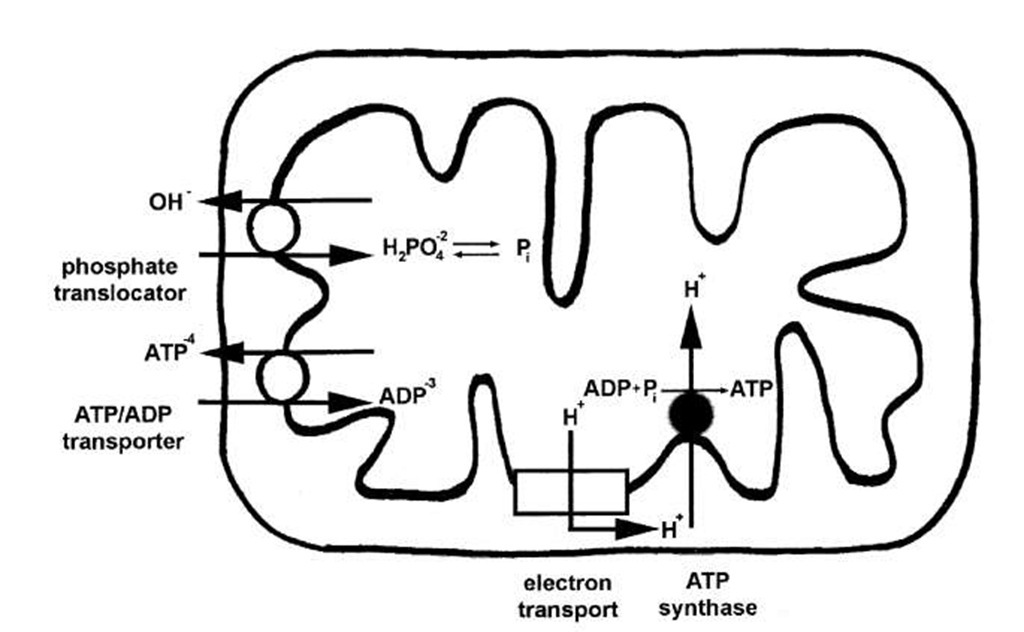
ATP is formed in the aqueous space bounded by the mitochondrial inner membrane. This space is known as the matrix (see Fig. 7). Most of the ATP generated within mitochondria is exported to the cytoplasm where it is used to drive energy-dependent reactions. The ADP and Pi formed in the cytoplasm must then be taken up by the mitochondria. The inner membrane contains specific proteins that mediate the export of ATP and the import of ADP and Pi. One transporter catalyzes counterexchange transport of ATP out of the matrix with ADP in the cytoplasm into the matrix (Fig. 8). At physiological pH, ATP bears four negative charges, and ADP, three. Thus, the one-to-one exchange transport of ATP with ADP creates a membrane potential that is opposite in sign of that created by electron-transport-driven proton translocation. ATP/ADP transport costs energy and the direction of transport is poised by the proton membrane potential. In addition, phosphate uptake into mitochondria is coupled to the electrochemical proton potential. The phosphate translocator (see Fig. 8) catalyzes the counterexchange transport of H2PO4— and hydroxide anion (OH-). The outward movement of OH-causes acidification of the matrix, whereas the direction of proton transport driven by electron transport is out of the mitochondrial matrix and results in an increase in the pH of the matrix.
In the total oxidation of glucose to CO2 and water, six CO2 are released and six O2 are reduced to water. For each pyruvate oxidized, four NADH and one FADH2 are generated. Since two molecules of pyruvate are derived by means of glycolysis from one molecule of glucose, a total of eight NADH and two FADH2 are formed by pyruvate oxidation. Four electrons are required for the reduction of O2 to two molecules of H2O. Thus, pyruvate oxidation accounts for the reduction of five of the six molecules of O2 in the complete oxidation of glucose. The sixth O2 is reduced to water by electrons from the NADH formed by the oxidation of triose phosphate in glycolysis.
FIGURE 8 ATP, ADP, and Pi transport in mitochondria. ATP is formed inside mitochondria. Most of the ATP is exported to the cytoplasm where it is cleaved to ADP and Pi. The mitochondrial inner membrane contains specific proteins that mediate not only ATP release coupled to ADP uptake, but also Pi uptake linked to hydroxide ion (OH-) release.
Fermentation, or anaerobic glycolysis, yields but 2 mol of ATP per 1 mol of glucose catabolized. In contrast, complete oxidation of glucose to CO2 and water yields about 15 times more ATP. Thus, it is understandable why yeasts and some bacteria consume more glucose under anaerobic conditions than when oxygen is present.
In animals, glucose is normally completely oxidized. During strenuous exercise, however, the demand for oxygen by muscle tissues can outstrip its supply and the tissue may become anaerobic. Muscle contraction requires ATP, and rapid breakdown of glucose and its storage polymer, glycogen, takes place under anaerobiosis. Glycolysis would stop quickly if the NADH produced by the oxidation of triose phosphate were not converted back to NAD+. In muscle cells under O2-limited conditions, pyruvate is reduced by NADH to lactic acid (see Fig. 5), a source of muscle cramps during exercise. At rest, lactic acid is converted back to glucose in the liver and kidneys and returned to muscle tissues where it stored in the form of glycogen.
C. Oxidation of Fats and Oils, Major Metabolic Fuels
Fats and oils are ubiquitous biological molecules that are major energy reserves in animals and developing plants. Fats and oils are esters of glycerol, a three-carbon compound with hydroxyl groups on all three carbons, and car-boxylic acids with long hydrocarbon chains. The most common fats and oils contain fatty acids with straight chains with an even number of carbon atoms. Most often, the total number of carbons in a fatty acid in a triglyceride ranges from 14 to 18. The difference between a fat and an oil is simply melting temperature. Oils are liquid at room temperature, whereas fats are solid. Familiar examples are olive oil and butter.
FIGURE 7 Diagrams of the structures of mitochondria and chloroplasts. The inner membrane of mitochondria and the thylakoid membrane of chloroplasts contain the electron transport chains and ATP synthases. Note that the orientation of the inner membrane is opposite that of the thylakoid membrane.
The most significant reason for this difference in melting temperatures between fats and oils is the degree of unsaturation (double bonds) of the fatty acids they contain. The introduction of double bonds into a hydrocarbon chain causes perturbations in the structure of the chain that decrease its ability to pack the chains closely into a solid structure. Olive oil contains far more unsaturated fatty acids than butter does and is thus a liquid at room temperature and even in the cold.
Regardless of the physical properties of triglycerides, they are the long-term energy reserves of higher organisms. Consider the fact that the complete oxidation of triglycerides to CO2 and water yields 9 kcal/g, whereas that of the carbohydrate storage polymers, starch and glycogen, yields just 4 kcal/g. When it is also remembered that fats and oils shun water, but glycogen and starch are more hydrophilic, triglycerides have an additional advantage over the glucose polymers as deposits of potential free energy. As hydrophobic moieties, fats and oils require less intracellular space than that required by the glucose polymers.
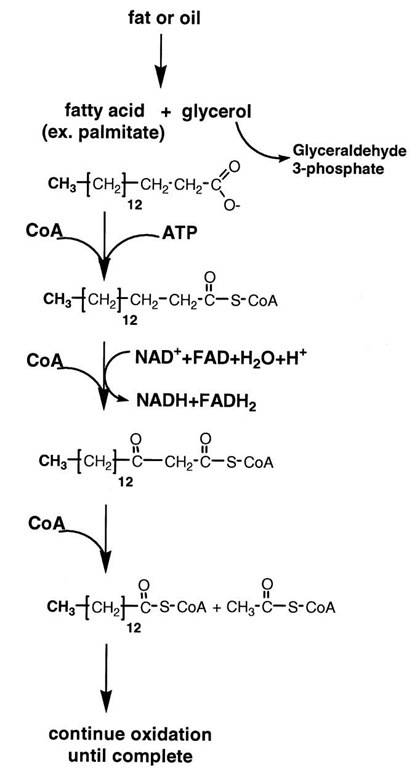
The first step in the breakdown of triglycerides (Fig. 9) is their conversion by hydrolysis to their components, glyc-erol and fatty acids. Glycerol is a close relative of the three-carbon compounds involved in the catabolism of glucose and may be completely oxidized to CO2 and water by glycolysis and the tricarboxylic acid cycle.
The fatty acids are first converted to CoA derivatives at the expense of the hydrolysis of ATP and then transported into mitochondria where they are broken down sequentially, two carbon units at a time, by a pathway known as fi-oxidation (see Fig. 9). The fatty acyl CoA derivatives undergo oxidation at the carbon that is fi to the carboxyl carbon from that of a saturated carbon-carbon bond to that of an oxo-saturated carbon bond. Enzymes that contain FAD or use NAD+ as the electron acceptors catalyze these reactions. As is the case in the oxidation of carbohydrates, the NADH and FADH2 generated by the fi-oxidation of fatty acids are converted to their oxidized forms by the mitochondrial electron transport chain, which results in the formation of ATP by oxidative phosphorylation.
Once fi-oxidation is complete, the terminal two carbons of the fatty acid chain are then released as acetyl CoA. Oxidation and cleavage of the fatty acid continue until it is entirely converted to acetyl CoA. The conversion of a saturated fatty acid with 18 carbon atoms to 9 acetyl CoA produces 8 NADH and 8 FADH2. The acetyl CoA is burned by the citric acid cycle to generate more ATP. The high caloric content of fats pays off to cells in the yield of ATP.
D. Catabolism of Proteins and Amino Acids
In addition to containing carbohydrates and fats, diets may be rich in proteins. The catabolism of proteins results in the generation of their component parts, amino acids. When the dietary amino acid requirements of an individual are satisfied, the excess amino acids in the diet are catabo-lized to CO2 and water as a source of energy. Some amino acids are degraded to molecules that feed directly into glycolysis, and others result in the production of acetyl CoA. Excess nitrogen resulting from the catabolism of amino acids and other compounds that contain nitrogen is excreted in mammals in urine in the form of the simple organic compound urea. Some amino acids are precursors in the biosynthesis of other organic molecules.
FIGURE 9 Oxidation of fatty acids. Fats and oils are hydrolyzed to form glycerol and fatty acids. CoA derivatives of the fatty acids are oxidized in mitochondria by NAD+ and FAD to fi-oxo-derivatives. CoA cleaves these derivatives to yield acetyl CoA and a fatty acid CoA molecule that is two carbons shorter. The process continues until the fatty acid has been completely converted to acetyl CoA. The acetyl moiety is oxidized in the citric acid cycle to CO2 and water. The complete oxidation of a fatty acid of about the same molecular weight of glucose yields four times more ATP than that of glucose.
E. Summary
In summary, organisms such as humans and other animals, many bacteria, fungi, and nongreen plants derive the energy they must have to power life from foodstuffs they obtain from their environment. The degradation of carbohydrates and the oxidation of fats are the major sources of energy for heterotrophic (other feeding) organisms. How these molecules that are essential to life are generated is the next subject considered.