13.12.
Low-maintenance and Maintenance Free Batteries
Use of improved materials and advanced constructional techniques have either reduced or eliminated the requirement of topping a battery periodically with distilled water to replace loss due to evaporation. The batteries without this maintenance are becoming attractive. These batteries incorporate the improved control of the charging rate, especially the voltage output, given by an alternator system as compared with a dynamo system. Gassing has been reduced by changing the grid material from lead-antimony alloy to an alloy of lead-calcium.
13.12.1.
Low-maintenance Type
This type of lead-acid battery (Fig. 13.69) requires less attention than the conventional type. When operated under normal temperatures and charged under suitable conditions, the electrolyte level of this battery requires checking only once per year, or after 80,000 km running.
The construction of a low maintenance battery is similar to a conventional type, except the change in the grid material from lead-acid antimony alloy to lead-calcium. Since the performance characteristics are based on proven designs, the battery can be used on vehicles as an alternative to the traditional type.
13.12.2.
Maintenance Free Type
Maintenance free battery incorporates several modifications over a conventional battery, the most significant feature is that it is sealed (except for a very small vent-hole) and requires no service attention other than to be kept clean.
The Delco-Remy Freedom maintenance free (Fig. 13.70) battery first appeared in America in 1971. In addition to being maintenance-free, this battery offers better cold weather starting power and improved resistance to heat and vibration damage.
The antimony has been eliminated from the plate grids, which could remove four major causes of early battery failure such as overcharge, water usage, thermal-runaway and self discharge. Thermal runaway is a condition, which occurs in a conventional battery when the battery operating temperature is high or when faulty regulation of the charging system is combined with a rising electrolyte temperature.
Overcharge is the major cause of gassing in a conventional battery. In a Freedom battery lead calcium (Pb Ca) is used for the grid material. Therefore with the inherent assistance of the higher emf given by this construction as it approaches full-charge, it is possible to reduce loss

Fig. 13.69. Low-maintenance battery (Lucas).
of water under overcharge conditions by over 80%. There still exists some gassing, and to encounter this gas reservoir is formed in the container to collect the water, which returns after cooling to the main electrolyte mass.
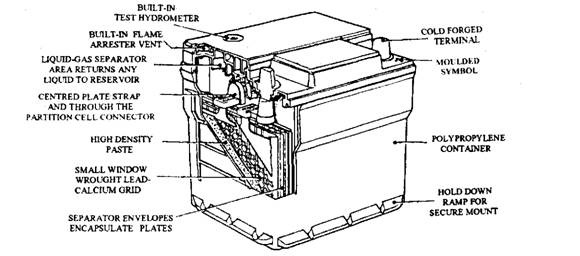
Fig. 13.70. Maintenance-free battery (Delco-Remy Freedom).
This battery incorporates a built-in, temperature-compensated hydrometer which indicates the relative density and level of the electrolyte. In fact various colours are displayed to indicate the states of charge. The appearance of a green-coloured signal means the battery is charged and usable, whereas a green/black or black signal indicates that recharging is necessary. When a light-yellow signal appears it indicates an internal fault so that the battery must not be charged or tested. Also when the battery is in this state the engine must not be started with jump leads. Instead a new battery should be installed in the vehicle and the alternator should be checked for correct operation.
If the battery is discharged to such an extent that it cannot crank the engine, and hence the engine has to be started by other means, then the alternator can not recharge the battery. When this condition arises the battery must be removed and bench-charged, because the voltage required to restore it is higher, which can not be provided by the charging system of the vehicle.
Other design improvements included over a conventional battery are strengthened grid supports, sealed terminal connections and stronger retention supports. These features together with a better efficiency enable this battery to be smaller and lighter than the conventional type.
Figure 13.71 illustrates a battery made by Chloride, called a ‘Torque Starter’. The Recombination Electrolyte (R.E.) is used in this maintenance-free battery to reduce the formation of oxygen and hydrogen when the battery is being charged. Each plate is wrapped with a glass micro-fibre separator that absorbs, in its pores, the entire liquid electrolyte. Therefore, there is no free acid in the cell unlike conventional batteries.
As the battery approaches its fully charged state, the oxygen generated at the positive plate passes through the separator pores to the negative plate. After initially reacting to form lead sulphate, the plate then changes to lead due to further charging. As a result of this action the negative plate never attains the right potential for the liberation of hydrogen, so that no water is formed. As no free oxygen or hydrogen is released, the battery is totally sealed, except for a

Fig. 13.71. Recombination electrolyte battery (Chloride Exide).
small pressure valve set, which opens if the battery is abused. The R.E. battery can deliver 20 percent more power than an equivalent conventional battery. Under the Cold Cranking Test the battery can deliver a current of 420 A. These features combined with better resistance to vibration and lightweight construction make the R.E. battery more attractive than the conventional type.
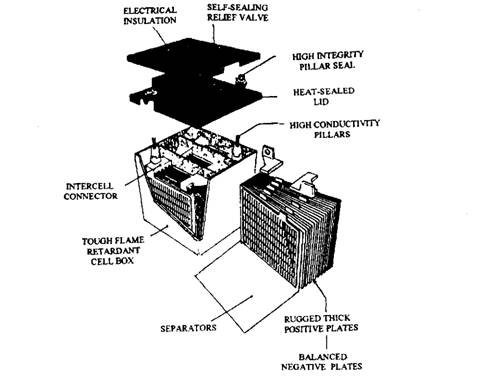
Fig. 13.72. A solid gel lead-acid battery.
Solid Gel Type.
Lead-acid batteries using a solid gel in place of liquid electrolyte are also manufactured. These batteries (Fig. 13.72) have many advantages, such as they do not leak and are more robust to withstand poor handling. A further advantage of a solid gel electrolyte is that a network of porous paths is formed through the electrolyte. If the battery is overcharged the oxygen emitted at the positive plate travels to the negative plate, where it combines with the lead and sulphuric acid to form lead sulphate and water.
![]()
This reforming of the water confirms that the battery is truly maintenance free. The recharging procedure is very similar to that used for conventional batteries.
One main problem with using a gel electrolyte is that the speed of the chemical reaction is reduced. Although this is not a problem for some types of supply, but the current needed by a vehicle starter is very high for a short time. Therefore, the ampere-hour capacity of this type of battery is often lower than the equivalent sized conventional battery. The solid-gel type electrolyte used in some types of these batteries is thixotropic. This means due to its high viscosity, the gel remains immobile even if the battery is inverted.
The gel type battery have not yet proved successful for normal motor vehicle application, but is an appropriate choice for specialist performance vehicles requiring an external power source for their starting.
