8.2.
Gasoline Fuel
Gasoline for automobile engines is called motor gasoline. Gasoline is usually a blend of several refinery products containing paraffins, naphthenes, and aromatics in varying proportions. The particular oiend depends upon the desired characteristics of the fuel. Because of high demand for gasoline, some of the smallest gaseous molecules are combined in refineries to form gasoline molecules and also of the large molecules are cracked to make gasoline. Modern techniques are capable of making more than one-half of each barrel of crude petroleum into gasoline.
Motor gasoline must meet the requirements of the engine. It must be light or sufficiently volatile to evaporate at low temperatures, for easy starting of the engine, but not so volatile as to evaporate in the fuel lines, causing vapour lock and thus preventing flow of liquid fuel. It also must not be so heavy that it does not evaporate or burn in the combustion chamber. If this happens, the unburned fuel runs down the cylinder wall, washing lubricating oil from the wall and diluting the motor oil. The volatility of the fuel is measured by a standard distillation test (Fig. 8.2). In this test, a 100 ml sample is heated in a distillation flask. The vapours are led through a condenser and the condensate is collected in a 100 ml graduated cylinder. The
temperatures of the vapours in the flask are recorded as each 10 ml is collected in the cylinder. The temperature-recovery curve is plotted on a distillation graph (Fig. 8.3). The most volatile parts of the gasoline evaporate at the lowest temperature, while the less volatile parts evaporate at higher temperatures. The distillation range of motor gasoline falls approximately within 308 to 478 K. The distillation curve is nearly the same for all motor gasoline sold in a geographic area regardless of the grade and brand.

Fig. 8.2. Apparatus to check gasoline volatility.
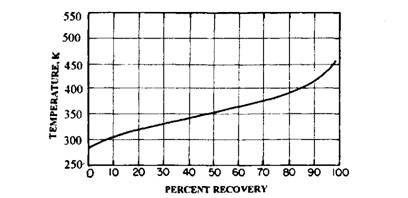
Fig. 8.3. Typical distillation graph for gasoline.
8.2.1.
Octane Number
The primary difference between gasoline grades is their antiknock quality. The octane rating is a scale that indicates the resistance of the gasoline to knock or detonation. The fuel octane number is determined in a standardised, single cylinder, variable-compression, fuel-research engine (Fig. 8.4). The engine is first adjusted to standard conditions while operating on a reference fuel. Under these standard conditions, the knock meter is adjusted to mid-scale. The sample fuel is then run in the resea :h engine under the same standard conditions as the reference fuel. Fuel mixture is adjusted to produce maximum knock, and the compression ratio is adjusted to produce the standard knock meter reading at mid-scale. Once all the conditions are kept constant, the engine is then run on reference fuel blend of know octane rating, one blend with slightly higher knock and one blend with slightly lower knock than the sample being tested. The sample fuel is assigned an octane number between the two reference blends. Different reference fuel bends, necessary to “bracket” the sample fuel, can easily be selected by referring to a table based on compression ratio that shows the approximate octane number. One primary reference fuel (PRF), isooctane, has been assigned 100 as an octane number and the other primary reference fuel, n-heptane, has been assigned ’0′ as an octane number. A blend of isooctane and n-heptane is used to test octane numbers below 100 octane; the octane number is given as the percentage of isooctane in the blend. For example, if the PRF blend contains 95% isooctane and 5% n-heptane, the blend has a 95 octane rating. Octane numbers above 100 octane can be tested by adding specific amounts of tetraethyl lead to isooctane to make reference fuel blends above 100 octane.
Fig. 8.4. Set up used to check the octane rating of gasoline.
Two different fuel research engines and test procedures are used to test motor gasoline. One procedure is called the Research Method and the other the Motor Method. The Research Method engine is run at 600 rpm with the inlet air temperature adjusted to compensate for barometric pressures. The Motor Method engine is run at 900 rpm and the air-fuel mixture temperature is held at 422 K. Gasoline produces a different octane number by each method and the Research Method usually gives a higher octane number. The difference between these two octane numbers is termed as the fuel sensitivity. The sensitivity of the fuel is due to the type of petroleum stock used to produce gasoline. The octane number displayed at gasoline pumping stations is the average of the octane number determined by these two methods.
8.2.2.
Octane Number Requirements
Gasoline has an octane number rating and each engine has a minimum octane requirement below which it does not run knock-free. The octane number requirement of the engine depends on the engine combustion chamber design, the operating mean effective pressure, the humidity of the charge, the temperature of the compressed charge, and the deposits present in the combustion chamber.
Two types of road tests are conducted to measure engine octane number requirement. The Modified Union town procedure determines the minimum octane gasoline required under the most severe operating conditions of the fuel and engine combination. The Modified Borderline procedure tests the engine along its entire operating range and can be duplicated on a number . of fuel samples. The Cooperative Research Council (CRC) selects a sample of automobiles each year to determine their octane requirements, and uses both test procedures to report engine octane requirements. Details of these two tests are different; however, in both tests the engine is operated on the sample fuel at specified speeds, using specified ignition timing. The knock intensity is determined by ear and is reported as borderline, trace, moderate, or heavy knock.
In these road tests, the engine is operated on a primary reference fuel blend (isooctane and n-heptane) which is close to the octane number and requirement expected. If the engine knocks more than required by the test procedure, the octane number of the reference fuel blend is raised and the test is repeated. If it knocks less than required, the reference fuel bend is lowered. The octane number requirement of the engines is equivalent to the primary reference fuel blend, which gives the specified knock intensity required by the test procedure.
8.2.3.
Desirable Properties
(a) Cheapness. The fuel should be cheap and readily available, but of f^ood quality.
(b) Compression Pressure. The fuel should have a high compression pressure limit without detonation to deliver more power.
(c) Flash Point. For safety reasons the flash point should be high for the fuel.
(d) Calorific Value. The greater the thermal energy of a fuel per unit volume and weight, the smaller is the quantity required to be carried in the tank in the vehicle to provide enough energy to transport a given load at a given speed over a given distance. High-density fuels contain the most energy and they have the lowest volatility.
(e) Latent Heat of Vaporisation. A high latent heat of vaporization of the fuel causes the charge to be cooled, and therefore becomes more dense, as the fuel mixes with the air. Under this condition the charge passing into the cylinders of the engine contains more energy than in the absence of that cooling. However, this can also cause freezing of atmospheric moisture in carburettor, which can severely affect the running of engines.
if) Boiling Point. The fuel must constitute a mixture of volatile liquids, called fractions, each having a different boiling point. This allows the engine to start easily on the more volatile fractions in the coldest winter conditions. Also, in very hot climatic conditions, it can still start and run satisfactorily on the heavier fractions at expected higher temperatures, without any problem due to the formation of bubbles of vapour, within the fuel system by the lighter in the heavier fractions. Good volatility in the middle range helps to reduce the duration of the use of the choke after start up from cold. Too high a proportion of heavy hydrocarbons can cause these heavy fractions to enter the cylinder in mainly liquid form, washing the lubricant from the cylinder walls and diluting it in the sump. Additionally, they tend to form heavy carbon deposits. Fuels are blended approximately for both seasonal and geographical variations in temperature.
(g) Purity. The presence of substances other than hydrocarbons in a fuel may cause deposit of ash or corrosive substances during burning or corrosion of components in the fuel system.
(h) Volatility. It is the tendency of the motor fuel to change from the liquid state to the vapour state. Ease of starting in cold conditions depends mainly on the volatility of the fuel. The more volatile the fuel, the more uniform is the distribution of the fuel in the cylinder and smoother the running of the engine. However, the main disadvantage of the high volatile fuels is that it vaporizes at normal temperature causing vapour lock in the fuel line and consequent failure of the engine to operate.
(i) Gum Content. Hydrocarbons and impurities in the fuel have a tendency to oxidise and form viscous liquids and solids causing the formation of gum. Lacquering is the phenomenon of formation of varnish appearing as residue left by the gum when exposed to high temperatures. When gum is inflamed it is reduced to a residue of carbon. Thus carbon, lacquer and gum deposits result from gum in the liquid fuel. A fuel with high gum content causes operating difficulties such as carbon deposits on sticky valves and piston rings, gum deposits in the manifold, clogging of carburettor, and lacquering of the cylinders, valve stem and pistons.
(J) Sulphur Contents. The fuel may contain free sulphur, hydrogen sulphide and other compounds of sulphur. Presence of sulphur and its compounds in the fuel damages manifolds and fuel pumps. During engine operation sulphur unites with oxygen to form sulphur dioxide which in the presence of water forms a mist of injurious sulphurous acids. Sulphur contents of less than 1% are acceptable.
(h) Anti-knock Quality. The fuel should have good antiknock quality. By adding a little quantity of tetraethyl lead, Pb (C2H5) to petrol the permissible compression ratio is raised considerably thereby reducing the tendency to knock. But lead oxide requires considerable energy for breaking up, so less energy is available for rapid combustion. A high octane number is perhaps the most important of all the properties required for a hydrocarbon fuel. With a low octane number, it burns explosively instead of progressively in the engine cylinders, and this can cause overheating and severe damage to the parts that are in any case very hot and their strength therefore reduces.
8.2.4.
Additives
Additives are basically chemicals mixed in very small proportions to the fuel to improve performance, enhance its desirable characteristics and to reduce the effects of its undesirable ones. The extent of their use depends on the circumstances in each different part of the world at any given time. The first additives were the anti-oxidants introduced at the early 1920s when the processes, such as cracking were known.
Lead Compounds.
The best-known additives are probably the lead compounds used for increasing the resistance of a fuel to detonation. Tetraethyl lead (TEL) was the first to be introduced in the early 1920s. In those days addition of about 0.6 g/litre TEL as a low cost way of increasing the octane rating of fuels, produced the maximum increase of 10 octane numbers depending on the nature of the base fuel. A level of about 0.3 g/litre is the lowest that can be accepted by engines not specifically designed for unleaded gasoline. Otherwise, less than this amount causes rapid wear of exhaust valves, which require a film of lead deposit on their seats to prevent local welding of the peaks of their surface texture.
By about 1960, tetramethyl lead (TML) having a similar effect came into use. This compound has a lower boiling point than TEL, and hence it evaporates with the lower fractions in the fuel. As a result, during acceleration, it is drawn preferentially into the cylinder to increase the anti-knock effect. However, due to the presence of lead in gasoline it becomes practically impossible to use catalytic converters to cleanse pollutants form the exhaust gases.
Alternative to Lead-oxygenates.
It is not an easy task to blend in the existing high-octane hydrocarbons such as the aromatics, since these constituents are in great demand for the production of petro-chemicals. But, higher-octane gasoline can be produced through catalytically reforming and isomerisation of the feedstock. These high-octane unleaded fuels are costly. On the other hand the use of low octane fuels in internal combustion engines having low compression ratios requires high rates of fuel consumption.
Another alternative is to blend in some oxygenates. These additives include alcohols such as methanol, ethanol, and tertiary butyl alcohol (TBA), which is 2-methyl-2-propanol, or (CH3)3 COH and certain ethers, the most commonly used among these is methyl tertiary butyl ether (MTBE), or C(CH3)3 OCH3, made from isobutylene (2-methyl-propane). All of these have high octane numbers. TBA is a simple isomer and the MTBE group is more complex.
But, alcohols absorb water and separate out with a layer of gasoline on top of a layer of alcohol-water mixture. Therefore they are not recommended in quantities above about 5.5% in the fuels supplied for distribution from service station forecourts. Methanol is the most unsatisfactory in this respect so, to diminish the problem, it has to be dissolved in a higher alcohol such as TBA. Some of theoxygenates have tendency to partially break down to form hydroperoxides which, although present in only very small quantities, are chemically active and therefore corrode or otherwise degrade some of the components in the fuel systems.
Anti-oxidants.
Similar to the rusting of iron in air, the oxidation of hydrocarbons and mono-olefins is very slow. However, traces of metallic salts including cobalt, copper, iron or manganese, if added to oil, they act as catalysts or as carriers of oxygen to accelerate the process. Copper is present in most crude oils and, after their refinement, may remain in the fuel in quantities between about 0.01 to 0.9 mg/litre. More may be introduced later due to arcing of the commutator in an immersed fuel lift pump delivering the fuel to the carburettor or injection equipment. Another source of addition of metallic salts, such as iron, zinc and nickel, is the corrosion by the peroxides on metal pipes and other components in the fuel system and storage facilities.
Amine anti-oxidants have been added in hydrocarbon fuels, in proportions between 8 and 40 parts per million (ppm). In the 1980s, the trend towards reduction of the olefinic content of
the gasolines from 20% to between 5 and 10%, the proportions of amines were reduced to between 5 to 10 ppm. The amines rapidly break down the hydroperoxides in fuels that contain alcohol. More widely used are the phenolic anti-oxidants, which retard the rate of decomposition into, hydroperoxides for periods exceeding the normal storage life of the fuel.
In some modern gasoline injection systems in which the gasoline is continuously circulated through fuel rails, and only a proportion drawn off for delivery to the injectors, the gasoline returning to the tank is warm. This causes a problem due to significant increase in the rate of formation of hydroperoxides and rapid exhaust of all the anti-oxidant present. Quantities recycled vary between 70 and 90%, depending on the load on the engine, which increase the rate of fuel consumption.
Detergent and Anti-icing Additives.
Detergent additives were added in the 1970s to keep clean the inlet valves and combustion chambers. Subsequently the others were introduced for cleaning the carburettor and its jets. These had to be added in carrier oil for satisfactory mixing with the gasoline. Such detergents were particularly useful in reducing atmospheric pollution. The practice was in use of removing blow by gases from the crankcase which otherwise had been discharged into the atmosphere through the crankcase breather. Presently these gases are discharged into the air intake, normally downstream of the air filter. Therefore, without detergent additives, these gases significantly increase the possibility of fouling of the components in the passages along which they are carried into the engine.
Glycols are added to gasoline to alleviate the problem of freezing of moisture in carburettors and induction systems. A problem due to deposits from the various additives themselves, particularly from the lead compounds, on both spark plugs and exhaust valve seats arose during mid 1950′s. These were countered by the use of phosphorous compounds. Subsequently due to the lowering of the lead content and improvements in spark plug design, these additives have become unnecessary. The precise nature of the many additives has become company secrets. Shell, for example, describes their detergent and phosphorous compounds simply by the initials ASD and ICA.
Drivability, Spark Aiders and Alcohol Blends.
Formula Shell is another of the additive packages, introduced in the early 1970s, before the advent of the energy crisis. Subsequently by about 1986, it was dropped because engine design itself could deal adequately with previous problems. Before the energy crisis, most engines used air-fuel ratios of about 13 :1 at throttle openings approaching idling and wide open. Afterwards the mixtures had to be weakened throughout the throttle range, falling to about 15 : 1 for cruising, as a measure to economize on fuel consumption and to help clean up the environment. With such weak mixtures, any wear or other in-service problem of carburation, injection or other equipment in the fuel system may cause serious effect on drivability.
Drivability can be said to be a measure of how smoothly the engine responds to signals transmitted to it through the accelerator pedal. It also includes the qualities of starting, idling and warm-up, as well as acceleration. The symptoms like hesitation, or a delay in response to the opening of the throttle ; stumble, or a drop in speed immediately after the throttle has been opened ; and surge, or an alternate gain and loss in speed at constant throttle opening are also included under drivability. A crucial factor influencing drivability is the speed with which the flame kernel initiated by the spark attains a critical size.
An engine running on a weak mixture is specifically prone to defective ignition, because of the difficulty of distributing the mixture in combustible proportions equally to each cylinder. On the other hand, given the richer mixtures of former times, even the cylinder receiving the leanest charge fires effectively and its contents burn rapidly. Therefore to strengthen and accelerate the development of the flame kernel, spark-aiders were introduced into the Formula Shell package.
Another requirement for drivability when using weak mixtures is better detergency and therefore, improved detergents were also introduced in the package. They were needed to prevent the build-up of gummy accumulations of carbonaceous deposits from the gasoline and lubricant in ports, manifolds, and combustion chambers and on valves. Such build-ups can cause an engine to need fuels of progressively increasing octane number as it ages. In extreme cases, this can happen in as little as 5000 km of running. The result can be uneven running, detonation, engine damage and poor fuel economy.
Entirely different problems can arise when high proportions of alcohols are blended with the conventional hydrocarbon fuels, as a measure to save national reserves of hard currencies. An unfortunate problem is that it reduces the effectiveness of conventional detergent additives, and as a result more suitable additives for such fuels are being found out.
8.2.4.
Gasoline Selection
Gasoline is generally available in premium, leaded regular, low-lead or no-lead grades. The main difference in the gasoline grades is the octane rating. Premium has the highest octane rating and is the costliest while no lead usually has the lowest octane rating and in some cases the cheapest. Other gasoline characteristics, such as volatility, vapour pressure, specific gravity, and cleanliness are practically equal, regardless of the gasoline grade.
Octane rating is an index of the ability of the gasoline to resist knock during combustion at high pressures and temperatures. If the engine does not knock on a low octane gasoline it is not useful to use a higher-octane gasoline. The higher-octane gasoline does not produce more power or run cleaner than the low octane gasoline in an engine designed to operate on low octane gasoline. When the engine knocks, a higher octane gasoline is necessary for the engine. An occasional use of low octane gasoline does not damage the engine if the operator reduces the throttle opening when knock is hard. By using different gasoline grades the operator can safely determine the lowest price gasoline that satisfies his engine. However, if the engine is not specified for the use of no-lead gasoline, then same should not be used. It is because there is possibility of valve failure under high power operation using no-lead gasoline. Similarly, if no-load gasoline is specified, then leaded gasoline should not be used in the engine, as it damages the catalytic converter.
All grades of gasoline have their volatility changed or adjusted by the oil companies throughout the year for the expected seasonal changes in temperature and geographical variations in altitude. The customer has no choice among the five volatility classes (A, B, C, D and E) that are provided for different months of the years and for different geographical locations. For the lowest fuel cost the operator should select the lowest price gasoline of the type specified for running knock-free in his automobile engine.
