8.5.
Gasoline Combustion
Vaporization of the hydrocarbons in gasoline and start of decomposition take place at temperatures below 593 K, which exist in the combustion chamber before the initiation of ignition. The products of combustion are mostly gases containing a large quantity of heat. The heat energy increases the gas pressure in the combustion chamber to produce the force on the engine piston, required to operate the engine.
The liquid gasoline must be converted to a vapour to burn in an engine. In carburetted engines vaporization of the gasoline must be done in one-third of a second at idle speeds and in one-thirtieth of a second at normal operating speeds. In fuel injected engines this must occur much faster. The carburettor during the process of mixing liquid fuel and air supports the vaporization process by breaking the liquid gasoline into sudsy foam that rapidly mixes with the air. The molecules of fuel and the molecules of oxygen in the air must combine in correct numbers. At sea level the air being dense a relatively small quantity is required for a given amount of gasoline. The air becomes less dense at high altitudes and at high atmospheric temperatures due to which the same volume of air contains a smaller number of oxygen molecules causing the air-fuel mixture to become richer in fuel. This causes problem on some emission controlled engines requiring leaner carburettor settings on automobiles used in the mountains than those used at sea level. Since automobiles are frequently operated in both mountains and at sea level, carburettors are provided with altitude compensation devices to prevent over-rich mixtures at high elevations.
When the charge is trapped in the combustion chamber, the molecules of oxygen in the air come into close contact with the hydrocarbon molecules of the gasoline. This causes rapid burning. A litre of gasoline if completely burned produces nearly a litre of water as well as sulphur dioxide in an amount dependent on the sulphur content in the gasoline. As the water is in a vapour form at normal operating temperatures it leaves the cylinder as a part of exhaust gas. When the engine is first started in cold weather condensed water vapour is visible in the exhaust. Condensed moisture with sulphur dioxide produces the acidic water, which is corrosive. During low temperature operating conditions such as suburban driving when the engine is cold, much of the moisture is condensed inside the engine. The combination of corrosion and wear under these conditions is the major reason for excessive wear of the top ring area of the cylinder wall.
8.5.1.
Normal Combustion
In a SI engine a homogeneous air-fuel mixture within the combustible range sustains the progress of a definite flame front across the combustion chamber, and combustion takes place in any location where fuel particle exists. In a CI engine, on the other hand, the air-fuel ratios in the various part of the chamber very widely, so no definite flame front is evident, and hence combustion occurs in many locations within the chamber.
A spark plug ignites the charge in the combustion chamber near the end of the compression stroke. The spark, produced across the spark plug electrodes at the correct time, must have sufficient energy to raise the gas temperature between the electrodes at a point so that the charge burning becomes self-sustaining. From this point, a flame front moves smoothly across the combustion. The flame front movement during normal combustion is illustrated in Fig. 8.6. Burning of charge takes place during approximately fifty degrees of crankshaft rotation due to which maximum force is exerted on the crankshaft. Actual combustion is much more complex and the combustion gases pass through many phases during the combustion process. For better understanding, the combustion is divided into two phases i.e. pre-flame reactions, and combustion.
As the gases are compressed and the temperature rises, pre-flame chemical reactions take place in the compressed charge thereby changing the character of the charge. These pre-flame reactions prepare the charge for burning.
As ignition takes place, depending upon combustion chamber turbulence the flame front moves out in a modified spherical fashion. The heat energy released behind the flame front increases combustion chamber pressure and temperature. Due to higher combustion chamber pressure and temperature the pre-flame reactions are increased in a portion of the charge, called the end gases, which remain ahead of the flame front. Pre-flame reactions increase more rapidly at higher engine compression ratios. If pre-flame reactions become too rapid, abnormal combustion takes place.
8.5.2.
Abnormal Combustion
Abnormal combustion may be divided into two main types i.e. knock or detonation and surface ignition. Each of these types causes loss of power and excessive temperature. Continued operation under either type of abnormal combustion gives rise to physical damage of the engine.
Detonation.
Engine knock or detonation is the out come of rapid pre-flame reactions within the highly stressed end gases. Due to the too rapid reactions spontaneous ignition of the end gases takes place as shown in Fig. 8.7. This causes very rapid combustion within the end gases, accompanied by high-frequency pressure waves. These waves hit the combustion chamber walls; as a result vibration noise sets which is called knock or detonation.
Detonation is affected by
(i) compression ratio,
(ii) the temperature and pressure at the end of compression, (Hi) the temperature of combustion chamber wall,
(iv) engine speed,
(v) fuel mixture strength,
(vi) combustion chamber shape,
(vii) the type of fuel,
(viii) ignition timing,
(ix) position of spark plug, and
(x) position of exhaust valve.
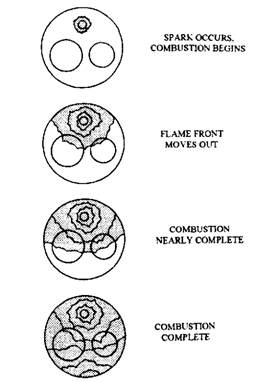
Fig. 8.6. Flame front movement during normal combustion.
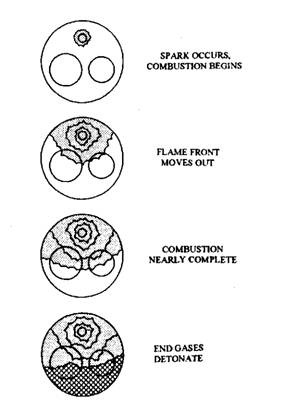
Fig. 8.7. Flame front movement during detonation.
The tendency of an engine to knock with a given fuel can be suppressed by lowering either combustion pressure or temperature, or both ; or by reducing the time the end gases are subjected to high pressures and temperatures. Also, using a fuel, which is less susceptible to rapid pre-flame reactions, reduces the tendency to knock. Octane rating is a measure of the anti-knock properties of a fuel. A fuel, which has high anti-knock characteristics, has a high octane rating.
Compression ratio has predominant effects on compression pressure. With the increase of compression pressure the output power of an engine increases. This is due to the higher combustion pressures, which are produced. High combustion pressures, however, increase the knock tendency. Fuels with high antiknock properties are used in higher-compression ratio engines to run engine knock-free while developing increased power. Lower compression ratios are used in low-emission engines so that they can run knock-free on low-octane unleaded gasoline.
Combustion chamber design also affects knock tendency. If combustion chambers end gases are in a squash or quench area, the engine has low knocking tendencies. This happens, as the
end gases are thin and close to a cool metal surface. Cooling the gases reduces and slows the end gas pre-flame reactions, thereby decreasing the engine knock tendency. This quenching of end gases is the main reason for a rotating combustion chamber engine to run knock-free on low octane gasoline.
Combustion chamber turbulence, as illustrated in Fig. 8.8, also helps to reduce knocking tendency by mixing cool and hot gases, thus preventing a concentration of static hot end gases where rapid pre-flame reactions can take place.

Fig. 8.8. End gases cooled in the quench area.
The detonation can be reduced by
(a) decreasing the combustion pressure and temperature,
(b) reducing the time the end gases are subjected to high pressures and temperatures,
(c) the use of fuel with a high octane number,

Fig. 8.9. Flame front movement during pre-ignition.
(d) proper design of combustion chamber where end gases are in a squash or quench area, and
(e) increasing combustion chamber turbulence.
Surface Ignition.
Surface ignition or secondary ignition, an abnormal combustion, starts at any source of ignition other than the spark plug. This is illustrated in Fig. 8.9. As surface ignition produces a secondary ignition source, its effect is to complete the combustion process sooner than normal, thereby developing maximum pressure at a wrong time in the engine cycle producing less power.
One potential source of secondary ignition is a hot spot, such as a spark plug electrode, a protruding gasket, a sharp valve edge, etc. These items can become extremely hot during engine operation forming a second source of ignition. These sources rarely occur in modern engine designs provided the engines are properly maintained. Another source of secondary ignition is combustion chamber deposits, which result from the type of fuel and oil used in the engine as well as from the type of operation of the engine. A deposit ignition source may be a hot loose deposit flake capable of igniting one charge before it is exhausted
from the engine with the spent exhaust gases. This is called wild ping. Sometimes, the flake remains attached to the combustion chamber wall. Under this situation, it ignites successive charges until the deposit is consumed or the engine operating conditions are changed.
When surface ignition occurs before firing of the spark plug, it is called pre-ignition. It may be audible or inaudible. It may be a wild ping or it may be a continuous runaway surface ignition. If it occurs after the ignition is turned off, it is called run-on or dieseling. Another phenomenon resulting from pre-ignition is engine rumble. Rumble is a low-frequency vibration of the lower part of the engine that occurs when the maximum pressure is reached earlier than normal in the cycle. Rumble has been almost eliminated from modern engines.
The knock-resistant fuels and antiknock additives generally tend to increase combustion chamber deposits thereby increasing the tendency to cause surface ignition. Fuel manufacturers therefore, use additional additives in the gasoline to reduce the deposit ignition tendency resulting from the antiknock additives deposits. Abnormal combustion seldom occurs in modern mass-produced automotive engines provided the recommended grade of fuel and motor oil is used and the engine is maintained and adjusted correctly. Some problems may exist in engines that are used exclusively for low-speed, short-trip driving. Abnormal combustion frequently occurs in engines modified for maximum performance and also some in emission controlled engines.
