8.7.
Chemistry of Combustion
Combustion is a chemical reaction in which heat and energy are produced. During the process of combustion a substance loses its own characteristics and changes to a new substance having altogether different physical and chemical properties. Fuels are having elements such as carbon and hydrogen, which are combustible and easily mixable with oxygen. Air is supplied for the provision of oxygen required for combustion.
Since air contains a large proportions of nitrogen, this gas is also induced. Nitrogen being an inert gas, however, does not enter into combustion process and thus appears as un-combined nitrogen in the products of combustion. The fuel combines with oxygen to produce CO2 and H2O. Therefore products of combustion, when burned in air consist of CO2, H2O, and N2. Since N2 always appears in the same quantity before and after combustion, it is disregarded in the chemical calculations for estimating the chemically correct, or stoichiometric air-fuel ratios for fuels. The combustion equations for some of the fuels are as below.
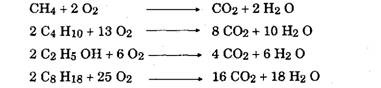
Carbon form the fuel combines with oxygen during combustion processes producing carbon dioxide. If, however, sufficient air is not provided, carbon monoxide is produced instead of carbon dioxide, which may reduce the quantity of heat to be produced during the combustion process. Hydrogen combines with oxygen to produce water. The chemical reactions for these are as follows.

Therefore, one kg of carbon needs (8/3) kg of oxygen for complete combustion, and produces (11/3) kg of carbon dioxide.
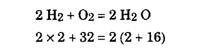
Therefore, one kg of hydrogen needs 8 kg of oxygen for complete combustion, and produces 9 kg of water.
![]()
Therefore, one kg of carbon monoxide requires (4/7) kg of oxygen to form (11/7) kg of carbon dioxide in the process of complete combustion.
The above analysis is by weight and is called gravimetric analysis. If the analysis is by volume, it is called volumetric analysis, which can be presented as below.
![]()
i.e. one m3 of hydrogen requires 0.5 m3 of oxygen to produce one m3 of water vapour. Similarly we have,
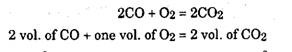
i.e. one m of carbon monoxide requires 0.5 m of oxygen to produce one m of carbon dioxide. The composition of atmospheric air as follows :
| Constituents | Molecular Weight | % by Volume | % by Weight |
| o2 | 32 | 21 | 23 |
| N2 | 28 | 79 | 77 |
| Air | 23.95 | 100 | 100 |
That means one kg of oxygen is supplied by (100/23) = 4.35 kg of air and one m3 of oxygen
is supplied by (100/21) =4.76 m of air. Therefore, knowing quantity of oxygen by weight or volume, the quantity of air can be calculated.
(a) Analysis by Volume form the Analysis by Weight. Let mi, m2, mz,… be the weight of the constituents and Mi, M% M3,… be the molecular weight of the above constituents.
![clip_image002[4] clip_image002[4]](http://lh4.ggpht.com/_Ii1ukGkfijY/SqqJsFHFZPI/AAAAAAAADek/f3u4zbYuP6Q/clip_image0024_thumb.jpg?imgmax=800)
This result is multiple by 100 for obtaining percentage by volume of each constituent.
(6) Analysis by Weight from the Analysis by Volume. Let vi, V2, v3,… be the volume of the constituents and Mi, M%, M3,… be the molecular weight of the above constituents. Then, viMi, V2M2, U3M3,… give the parts by weight of the constituents respectively.
![clip_image004[4] clip_image004[4]](http://lh5.ggpht.com/_Ii1ukGkfijY/SqqJufQla7I/AAAAAAAADes/vHyJ22XMa68/clip_image0044_thumb.jpg?imgmax=800)
This result is multiplied by 100 for obtaining percentage by weight of each constituent.
(c) Weight of Exhaust Gases per kg of Fuel. Since air is supplied for combustion, weight of exhaust gases is more than the weight of fuel burned. The actual weight of dry flue gas can be obtained by comparing the weight of carbon in the flue gases with the weight of carbon in the fuel, as there is no loss of carbon during the process of combustion. In case some unburned carbon is present in the exhaust gases, the weight of the same is to be subtracted from the weight of the carbon in the fuel. The analysis of flue gases is generally volumetric, which has to be converted first in the analysis by weight.
Then weight of exhaust gases per kg of fuel burned
![]()
(d) Weight of Excess Air Supplied. The weight of excess air supplied can be obtained from the weight of unused oxygen found in the exhaust gases after considering, if any, carbon
monoxide in the exhaust that has been burnt to form carbon dioxide using oxygen. This weight must be converted to the weight per kg of fuel burned.
Weight of excess oxygen per kg of fuel = Weight of excess oxygen per kg of fuel gas x weight of fuel gas per kg of fuel.
Hence weight of excess air supplied = Weight of oxygen x (100/23).
Let At = Theoretical amount of air required for complete combustion of fuel in the engine.
A = Actual amount of air supplied to the engine. Then, a = A/At, called excess air coefficient.
In park ignition engine ‘a’ may be greater than one (weak mixture) or smaller than one (rich mixture) and ‘a’ changes from 0.85 to 1.15. In Diesel engines ‘a’ is always greater than one and it changes from 1.15 to 5 depending on load.
Example 8.1. The composition of petroleum by weight gives 86% of carbon and 14% hydrogen. Calculate the weight of air required for complete combustion of one kg of the fuel. Also estimate the composition of the products of combustion.
![clip_image002[6] clip_image002[6]](http://lh6.ggpht.com/_Ii1ukGkfijY/SqqJy9JcpXI/AAAAAAAADe8/IzvS2pj2Xnc/clip_image0026_thumb.jpg?imgmax=800)
Example 8.2. Theoretical amount of air required for the complete combustion of fuel is 15 kg. If it is composed of carbon and hydrogen only, find the percentage composition of its constituents.
![clip_image004[6] clip_image004[6]](http://lh6.ggpht.com/_Ii1ukGkfijY/SqqJ0jGBcTI/AAAAAAAADfE/eAlckfg3O5w/clip_image0046_thumb.jpg?imgmax=800)
