Mysida
(Mysids)
Phylum Arthropoda
Subphylum Crustacea
Class Malacostraca
Number of families 4
Thumbnail description
Small shrimplike crustaceans with a flexible carapace enveloping the thoracic region along the sides; stalked eyes; and a well-developed tail fan
Photo: The glass shrimp Mysis relicta is found in freshwater.

Evolution and systematics
Fossil mysids have been dated as far back as the Triassic period, about 248-213 million years ago (mya). A group of fossil crustaceans known as Pygocephalomorpha, which includes a number of Paleozoic genera from the Carboniferous and Permian periods (360-248 mya), is possibly related to the mysids.
Two suborders for the order Mysidacea, the Lophogastrida and Mysida, were recognized in 1883. This arrangement persisted for nearly a century until some researchers proposed alternative taxonomic schemes to explain the relationships among mysidaceans, including raising both suborders to order level. This proposal, which has been sustained by a number of experts, is followed here.
The order Mysida includes four families: Petalophthalmi-dae, with six genera; Mysidae, with six subfamilies (one, the Mysinae, comprises seven tribes) and almost 140 genera; Lep-idomysidae, with only one genus, Spelaeomysis; and Stygiomysi-dae, also with only one genus, Stygiomysis. The order as a whole includes slightly over 1,000 described species.
Mysids are sometimes known as opossum shrimps because of the marsupium, or external pouch, formed by specially developed plates on the inner sides of the thoracic limbs of adult females.
Physical characteristics
Most mysids are fairly small, between 0.39 and 1.18 in (10 and 30 mm) long. They have a shieldlike carapace that covers the cephalon (head region) and most of the thorax. The carapace is fused with the first three (in rare cases the fourth)
thoracic somites (segments). The eyes are usually stalked and movable; the cornea is generally developed with visual elements, but tends to be reduced with increasing depth of habitat. The antennules (antenna 1) and antennae (antenna 2) are biramous (forked). Male mysids typically bear a setose (bristly) lobe, the processus masculinus, on the peduncle of the an-tennules.
The thorax has eight pairs of pereopods or thoracic limbs, all of which are divided into two branches, an endopod and an exopod. The endopods of the first and sometimes the second pereopod are usually transformed into gnathopods (specialized appendages for feeding), which differ considerably from the remaining limbs. Female mysids have a marsupium made of fewer than seven pairs of lamellae (oostegites or brood plates), in which the embryos are kept until they grow into juveniles. The abdomen consists of six somites, generally similar in form but with the last somite longer than the others. Each of the first five abdominal segments bears a pair of pleopods. The pleopods are biramous, frequently reduced in the female and sometimes in the male. They are often sexually modified in the male. The telson, or posterior extremity of the body, has a pair of appendages known as uropods, which form a well-developed tail fan. A statocyst, which is a tiny organ related to the animal’s sense of balance, is usually present in the endopod of the uropods.
Mysids themselves are often glassy or transparent; they can be seen only when the observer notices their black eyes darting about in the water. Most mysids have a body pattern formed by dark star-shaped chromatophores (clusters of pig-mented cells) against a light background color. Some species turn dark when placed against a black background; others that are usually light green and found among green algae may change to dark olive. Deep-sea mysids are often red.

Close-up of the head of the species Stygiomysis cokei.
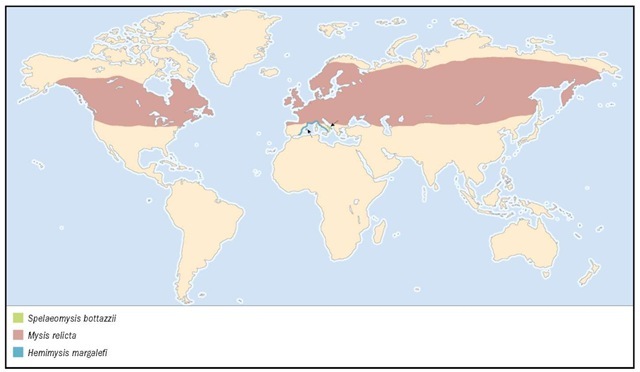
Distribution
Mysids are widespread over all continents. They live in a variety of aquatic environments, including coastal and open sea waters, estuaries and other brackish water ecosystems, and continental freshwater lakes and rivers. In addition, a few species have been found in different groundwater habitats and in anchialine (from Greek words that mean “near the sea”) caves, which are coastal caves formed from limestone or volcanic rock that are flooded with seawater.
Habitat
Mysids are originally marine crustaceans. They are a highly adaptive group, however, which makes them effective invaders of new habitats, including brackish water and freshwater environments. As a whole, the group is essentially pelagic, although commonly epibenthic, which means that they live on or immediately above the surface of the sediment. Some species burrow into the sediment, live just above the sandy or muddy bottom, or migrate between substrates at the bottom and the surface waters. A few are strictly pelagic species; some live in shallow water in the littoral zone among macroalgae, in crevices along rocky shores, or on sandy beaches. Many species of deep-sea mysids are found on or just above the ocean floor at various depths, including abyssal waters at depths of 18,700-23,622 ft (5,700-7,200 m).
Behavior
Most sand-burrowing and coastal mysids perform a diel (24-hour cyclical) vertical migration, rising and dispersing into the water column at night and returning to deeper water towards dawn. Many are benthic by day and pelagic at night. A few species rest on algae during the day or on stones and cliff ledges; only a few mysids bury themselves in sand. Gastrosaccus throws up sand grains by moving its thoracic limbs while lying on the sand. Paramysis digs ditches in muddy sand with its first three pereopods. This species can dig for long periods of time, producing open ditches as long as 1.9-3.9 in (5-10 cm) within an hour.
Almost all bathypelagic and bottom-dwelling mysids, even those that burrow in sand, rise in the water column at night. The stimulus for this migration may be light intensity; the time it takes the mysids to rise depends on the speed and depth of water currents. This nocturnal pattern is most noticeable during spawning periods, and may be related to the dispersal of young mysids leaving the marsupium. Littoral species sometimes move to deeper water in fall and return to the shoreline in spring or summer.
Some species of mysids form swarms. These swarms may be several miles long and three or more feet in diameter.
Mysids are primarily swimmers; all members of the class can swim up, down, forward, and backward. The females have reduced pleopods and swim with the exopod (external branch) of their pereopods. They hold the exopods out to the sides and rotate them so that the tip describes an oval. They move their limbs continuously in a slightly different phase and draw water from the sides toward their upper surface. In this way two strong currents of water, one parallel to the abdomen and the other some distance from it, drive the body forward. Many species hold their bodies in a horizontal position with the dor-sum up while swimming. A few hold the anterior part of their bodies in an almost vertical position.
Some species swim in schools. School formation among mysids depends on optic signals during the day and probably sensitivity to water currents generated by the swimming movements of their neighbors at night.
Mysids that are suddenly disturbed jerk backward by flexing their abdomen and tail fan against their thorax. Bottom dwellers walk slowly on their endopods with their exopods also in constant motion. The eyes and statocysts keep the body horizontal even in the dark. If a mysid is illuminated from the side, it will turn its back toward the light. The angle of turning may be greater than 45° especially if the light is strong. The animal’s optic control over body position is able to override the statocysts.
Feeding ecology and diet
Most mysids are filter feeders, removing fine detritus, rotifers, mollusk larvae, diatoms, and other planktonic organisms out of the water while swimming just above the bottom and creating a suspension feeding current. All filter-feeding mysids may also feed raptorially; that is, they may actively capture selected prey from the environment. They have species-specific feeding modes; some species can switch from one feeding mode to another according to food availability.
Members of some mysid genera, including Neomysis and Siriella, have been seen to catch small live crustaceans (cope-pods, cladocerans, amphipods) as well as small mollusks. Mysids use their gnathopods to seize and feed on zooplankton as well as to strain phytoplankton and particulate debris. These appendages move the food under the animal’s mandibles (jaws) and press it against these cutting appendages.
On the other hand, mysids are the prey of many larger predators around the world, including invertebrates, fishes, birds, seals and whales.
Reproductive biology
Male mysids do not actively search for females during reproduction. After shedding a previous brood, the female soon molts and is ready to breed again. At that time she produces a pheromone, or chemical substance that stimulates the an-tennules or the antennular processus masculinus of nearby males. Mating is very quick and takes place at night. The male lies under the female either head-to-tail and belly-to-belly, or doubles up and grasps the anterior part of the female’s abdomen with his antennae. The sperm are either injected into the female’s brood pouch or shed between the mating individuals and swept by currents produced by the thoracic appendages into the marsupium. The copulating pair soon separate, and within half an hour the female’s eggs are extruded into her brood chamber and fertilized there.
The incubation period and frequency of mating depends on the species and the water temperature, and can range from a few weeks to several months. The young are shed as juveniles with complete sets of appendages. Released mysids need about a month to reach their adult stage at a water temperature of about 68°F (20°C).
Conservation status
The IUCN has placed three species, all anchialine stygo-bitic mysids (Bermudamysis speluncola, Platyops sterreri, and Sty-giomysis hydruntina) on its Red List as Critically Endangered and another one as Vulnerable.
Regression or even complete extinction of mysid populations as a result of human impact has been documented. Mysids are endangered by domestic and industrial pollution of coastal waters; dredging of canals (for land use, fisheries, and navigation); artificial redirection of waters (for river traffic and dykes); groundwater drainage; and the repeated application of pesticides. Subterranean ecosystems are variously threatened by tourism, agriculture, urbanization, and the construction of hydroelectric reservoirs.
Significance to humans
Some mysids are used as fish food in commercial aquacul-ture. On the Island of Jersey, mysids are compounded into a paste called “cherve,” which is sold to mullet anglers for bait. In the Orient, mysids are harvested commercially for human consumption. In Japan, Neomysis intermedia and N. japonica are used for tsukudani, a popular dish made with soy sauce.
Mysids are also used for scientific research. They are excellent experimental organisms because they are easy to collect, relatively easy to handle, and stay healthy in the laboratory for long periods of time.

1. Amathimysis trigibba; 2. Stygiomysis cokei; 3. Mysis relicta; 4. Hemimysis margalefi; 5. Spelaeomysis bottazzii.
Species accounts
No common name
Spelaeomysis bottazzii
FAMILY
Lepidomysidae
TAXONOMY
Spelaeomysis bottazzii Caroli, 1924, caves near Castro Marina, Otranto, Italy.
OTHER COMMON NAMES
None known.
PHYSICAL CHARACTERISTICS
This mysid has a short carapace with the last two thoracic somites exposed on the dorsal surface. The rostrum is broadly rounded and the eyes reduced in size. Antennal scale (a lobelike modification of the external branch or exopod of antenna 1) small, without apical suture, outer margin smooth, setose, without terminal spine. The second thoracic limb (pereopod) has developed as a gnathopod; the exopod is well developed. The marsupium in the adult female is composed of seven pairs of oostegites. The sixth and seventh abdominal somites are fused.
DISTRIBUTION
Southern Italy in the Salentine Peninsula around Lecce; the caves of Zinzulusa, Buco dei Diavoli and L’Abisso; artificial wells near Gallipoli and the area around Bari; groundwaters around Gargano.
HABITAT
This mysid lives in brackish underground waters. It is a eury-haline (tolerant of a wide range of salt concentrations) and eu-rythermal (adaptable to a broad range of temperatures) species that can live in darkness as well as full or dim light.
BEHAVIOR
Spelaeomysis bottazzii has been kept alive for more than four months in the laboratory under different lighting conditions, in fresh as well as salt water, with temperatures ranging from 50°F-64°F (10°C-18°C).
FEEDING ECOLOGY AND DIET
This species feeds on diatoms and other autotrophic (self-nourishing) aquatic microorganisms.
REPRODUCTIVE BIOLOGY
Spelaeomysis bottazzii has a long period of development in the marsupium as well as a particular reproductive strategy that is not known in other mysids. Females of this species assume an immature form after molting at the end of the incubation period, probably in order to build up nutrition reserves before starting a new reproductive cycle.
CONSERVATION STATUS
This species is known from fewer than five localities scattered over an area of less than 38.6 square miles (100 square kilometers) in southern Italy. The IUCN Red List categorizes it as Vulnerable.
SIGNIFICANCE TO HUMANS
None known.
No common name
Amathimysis trigibba
FAMILY
Mysidae
TAXONOMY
Amathimysis trigibba Murano and Chess, 1987, Isthmus Reef, Catalina Island, California, United States.
OTHER COMMON NAMES
None known.
PHYSICAL CHARACTERISTICS
The size range for adult specimens is 0.1-0.13 in (2.7-3.5 mm). The general form is sturdy. The frontal margin of the carapace leads to a broadly rounded rostral plate. The side margins of the rostrum are evenly convex, partially covering the eyestalks in females. The eyestalks are exposed in males. The posterior margin of carapace leaves the last thoracic somite exposed. There are three tubercles (nodules) on the dorsal surface located between the frontal margin of the carapace and the cervical groove. The eyes are developed, and slightly longer than broad; the cornea is wider than the stalk. The antennal scale has a long apical lobe. Female marsupium has two pairs of oostegites, with the posterior pair considerably larger than the anterior pair. Telson entire, 1.4 times as long as broad; lateral margins naked, proximal two-thirds convex and distal one-third concave; distal margin transverse, one-fifth of maximum width at base, armed with 2 pairs of spines, inner pair two-fifths of telson length.
DISTRIBUTION
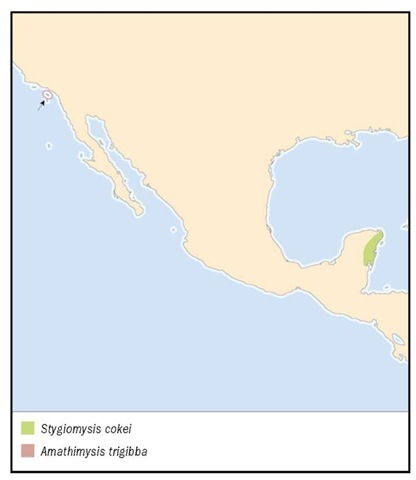
Catalina Island, Pacific coast of California, United States.
HABITAT
Found at a depth of 16.4-75.4 ft (5-23 m). Most abundant within the suspended detritus layer just over the sandy substrate, but also found among low benthic algae growing on rocks.
BEHAVIOR
Rises at night several feet into the water column where it occurs in densities of about 8.5 per m3.
FEEDING ECOLOGY AND DIET
Nothing is known.
REPRODUCTIVE BIOLOGY
Nothing is known.
CONSERVATION STATUS
Not listed by the IUCN.
SIGNIFICANCE TO HUMANS
None known.
No common name
Hemimysis margalefi
FAMILY
Mysidae
TAXONOMY
Hemimysis margalefi Alcaraz Riera, and Gili, 1986, dark submarine cave on the northeastern coast of Mallorca in the western Mediterranean, 39°45N, 3°26′E, at a depth of 39.3 ft (12 m) depth.
OTHER COMMON NAMES
None known.
PHYSICAL CHARACTERISTICS
Living specimens are bright red in color. The total size of an adult specimen from the anterior margin of the carapace to the distal end of telson ranges from 0.16 to 0.21 in (4.12-5.49 mm). General form robust. Carapace short, emarginate posteriorly, thoracic somites 6 and 7 exposed dorsally; anterior margin forming a broad angle rostrum; cervical groove present. Eyes large, globular, slightly broader than the eyestalk, extended laterally beyond the limits of the carapace; cornea pigment black. Antennal scale lanceolate, narrow, about 5.5 times as long as broad in the broadest part; outer margin straight or slightly curved outward; the proximal 1/5 of its length naked (without a spine or a tooth). Telson short; lateral margins converging distally, armed with 8-11 spines; width at distal end less than half the broadest part; telson cleft for about 1/5 of its length, cleft armed with 20-30 small teeth; apical lobes with a long, strong spine in the distal end.
DISTRIBUTION
Mediterranean; northeastern coast of Mallorca.
HABITAT
Dark submarine caves.
BEHAVIOR
This species demonstrated adaptability to a range of temperatures and resistance to acute thermal stress during a series of laboratory experiments.
FEEDING ECOLOGY AND DIET
Nothing is known.
REPRODUCTIVE BIOLOGY
Nothing is known.
CONSERVATION STATUS
Not listed by the IUCN.
SIGNIFICANCE TO HUMANS
This species is being used to study the physiology of mysids.
No common name
Mysis relicta
FAMILY
Mysidae
TAXONOMY
Mysis relicta Loven, 1862, cold water lakes in northern Europe.
OTHER COMMON NAMES
None known.
PHYSICAL CHARACTERISTICS
The total size of mature specimens is 0.59-0.98 in (15-25 mm) long. The antennal scale is only four times as long as it is wide. The exopod on the third pleopod of mature males is well developed and has five segments while the exopod on the fourth pleopod has six. The telson has a wide bifurcated tip.
DISTRIBUTION
Mysis relicta has a circumpolar distribution. It is found throughout the northern latitudes (above 42°N) in the Great Lakes of North America; Green Lake, Trout Lake, and Lake Geneva in Wisconsin; the Finger Lakes of New York; a handful of Canadian shield lakes; Europe, Scandinavia, and Russia. Considered a relict (survivor) of the great glaciers of the Pleistocene Epoch (1.8 million years ago), its natural distribution has been increased by its introduction into continental waters as an addition to the forage base of sport fisheries.
HABITAT
Cold freshwater. During warm months almost restricted to temperatures range as low as 39.2°F (4°C). It never lives in waters warmer than 57.2°F (14°C). In northern Germany this species does not occur in water with an oxygen concentration lower than 4 cmVliter; in Wisconsin, however, it lives in water with an oxygen concentration as low as 1 cm3/liter.
BEHAVIOR
Members of this species are swimmers, remaining in deep cold water during summer and moving in winter to shallower waters to reproduce. They remain near the bottom during daytime and rise at dusk toward the surface to forage. Smaller juveniles often lead the migration, rise higher in the water column than the adults, and are the last to descend. Adapted to living in dark environments, their eyes are easily damaged by strong light.
FEEDING ECOLOGY AND DIET
Mysis relicta is an opportunistic feeder with both filter-feeding and predatory habits. It feeds mainly on detritus stirred up from the bottom, including diatoms and unicellular algae. The remains of small crustaceans (cladocerans and copepods) have also been found in its stomach contents, however, which indicates that it also feeds heavily on zooplankton and carrion.
REPRODUCTIVE BIOLOGY
Brood size is positively related to the female’s size. A female 0.51-0.59 in (13-15 mm) long carries 10-20 embryos while a female 0.66-0.82 in (17-21 mm) long carries as many as 25-40 embryos. The young are carried in the brood pouch for 1-3 months and leave the marsupium when they are (3-4 mm) long. Mature individuals are 0.51 in (13 mm) long. M. relicta lives for over a year and as long as two years, reproducing once or twice before it dies.
CONSERVATION STATUS
Not listed by the IUCN; often introduced as important food item for forage fish.
SIGNIFICANCE TO HUMANS
Mysis relicta is commercially important to fisheries managers as prey for a large number of coldwater fishes, including lake trout, brown trout, rainbow trout, kokanee salmon, various coregonids, burbot, smelts, and alewives. ♦
No common name
Stygiomysis cokei
FAMILY
Stygiomysidae
TAXONOMY
Stygiomysis cokei Kallmeyer and Carpenter, 1996, Temple of Doom Cave (328 ft [100 m] in from cave entrance), 3.7 mi (6 km) northwest of Tulum, Quintana Roo, Mexico, at a depth of 32.8-65.6 ft (10-20 m).
OTHER COMMON NAMES
None known.
PHYSICAL CHARACTERISTICS
The size of this mysid ranges from 0.35 to 0.86 in (9.0-22.0 mm). The length of the colorless wormlike body is 7.0-7.2 times the width. The length of the carapace is about one-fifth the length of the body. The abdominal somites are smooth and rounded on the dorsal surface. Antenna 1 is about one-half the length of the body. Four paired ventral lamellae or oostegites (typical for Stygiomysis females and a number unique among the Mysida) extending medially and anteriorly from the proximal part of pereiopods 3-6; each oostegite single flexible membranous flap, rounded and elongated anteriorly. The length of the telson is about 1.7-2.0 times its width, or one-sixth the length of the body. It has 15 spines arranged in five groups of three on its posterior margin.
DISTRIBUTION
Coastal inland caves, Quintana Roo, Yucatan Peninsula, Mexico; the type locality, Mayan Blue Cave; Carwash Cave; and Naharon Cave.
HABITAT
These mysids are stygobites, or cave dwellers. All caves from which this species was collected are completely underwater and entered through water-filled limestone sinkholes. The specimens were collected at depths of 32.8-65.6 ft (10-20 m) in the freshwater layer, occasionally in the upper part of the halocline (a well-defined vertical gradient of salinity). Conditions remained relatively constant with temperatures around 76.1°F-77.9°F (24.5°C-25.5°C), pH 6.8-7.0, low oxygen (near 2.0 ppm), and high carbon dioxide levels (44-864 ppm).
BEHAVIOR
The behavior of 5. cokei was observed in a laboratory setting. Open containers of cave water readily lost carbon dioxide, causing the pH to rise. The mysids kept their tails almost straight up at a right angle when the pH was comfortably low. As the pH rose, their tails gradually dropped in proportion to the increase; in extreme conditions, their tails were nearly horizontal. They also lowered their tails to a nearly horizontal position while walking. When the animals were forced off their substrate in the caves or the laboratory, they displayed frantic and ineffective swimming movements. The uropods spread away from the tel-son to make a wide tail fan when 5. cokei is walking. In healthy specimens, the respiratory beating of pereiopods 1-7 occurred in sequences of 3-9 seconds, followed by rest periods that lasted 2-45 seconds. As carbon dioxide levels dropped and the pH rose, their rest periods increased to as long as 50 minutes.
FEEDING ECOLOGY AND DIET
Primarily filter feeders.
REPRODUCTIVE BIOLOGY
Nothing is known.
CONSERVATION STATUS
Not listed by the IUCN.
SIGNIFICANCE TO HUMANS
None known.
