Definitions
Urinary tract infection (UTI) is the most common of all bacterial infections; it affects persons throughout their life span. The term UTI encompasses a variety of clinical entities, ranging from asymptomatic bacteriuria to cystitis, prostatitis, and pyelonephritis. UTIs may be further characterized as uncomplicated (occurring without an anatomic or other predisposing reason) or complicated (associated with structural or functional abnormalities of the urinary tract and kidney) and as community acquired or nosocomial (generally, catheter associated).
Epidemiology and Risk Factors
UTI occurs far more commonly in females than in males, except at the extremes of age [see Table 1]. During the neonatal period, the incidence of UTI is slightly higher in males than in females because of the greater frequency of congenital anomalies of the urinary tract in male infants. After 50 years of age, the incidence of UTI is almost as high in men as in women, presumably because of obstruction from prostatic hypertrophy. In persons between 1 and about 50 years of age, UTI is predominantly a disease of females.
Women
As many as 50% to 80% of women in the general population acquire at least one UTI during their lifetime; most of these infections are uncomplicated cystitis.1 In a prospective cohort study of sexually active healthy women, the incidence of acute cystitis was 50 to 70 episodes per 100 person-years.2 Recent use of a diaphragm with spermicide, frequency of sexual intercourse, and a history of UTI were identified as independent risk factors for cystitis in this study.2 Cystitis has also been temporally related to recent sexual intercourse.
The incidence of acute uncomplicated pyelonephritis is difficult to ascertain because this infection is less common than cystitis and because most episodes are treated in the outpatient setting. In a population-based case-control study of young healthy women, the annual incidence of pyelonephritis was approximately 28 per 10,000 women. Factors independently associated with pyelonephritis in this study included frequency of sexual intercourse, having a new sexual partner, UTI in the past 12 months, maternal history of UTI, diabetes, and incontinence.
Table 1 Incidence of Urinary Tract Infection According to Age and Sex
|
Age Group |
Incidence (%) |
Approximate Sex Ratio (Male:Female) |
|
Neonatal |
1.0 |
1.5:1.0 |
|
Preschool age |
1.5-3.0 |
1:10 |
|
School age |
1.2 |
1:30 |
|
Reproductive age |
3-5 |
1:50 |
|
Geriatric |
10-30 |
1:1.5 |
Thus, many of the factors predisposing women to cystitis also increase the risk of pyelonephritis.
Young men
UTI is rare in young men and has traditionally been attributed to the presence of urologic abnormalities. However, it is apparent that uncomplicated UTI can occur in men who acquire uropathogens through direct sexual contact, in the form of unprotected anal intercourse with a man or a woman, or unprotected vaginal intercourse with a woman whose vagina is colonized with uropathogens.4 Lack of circumcision is also associated with an increased risk of UTI, because of an increased incidence of Escherichia coli colonization of the glans and prepuce and the subsequent migration of E. coli to the urinary tract.56
Recurrent uti
About 20% to 30% of women who have had one episode of UTI will have recurrent episodes.4 Recurrence may result from relapse or reinfection. Relapse in either sex is caused by the reappearance of an organism from a sequestered focus, usually within the kidney or prostate, shortly after completion of therapy. Sequestration of infecting organisms in the bladder epithelium has been demonstrated in animals, but the importance of this phenomenon in humans is not yet clear.7 In reinfection, the course of therapy has successfully eradicated the infection and there is no sequestered focus, but organisms are reintroduced from the fecal reservoir. The majority of recurrences are thought to be rein-fections.4 Studies of the natural history of recurrent UTI in women have found that the rate of recurrence ranges from 0.3 to 7.6 infections per patient per year, with an average rate of 2.6 infections per year.8 Clustering of episodes occurs; it is not uncommon for multiple recurrences to follow an initial infection. The likelihood of a recurrence decreases with increasing time since the last infection. A case-control study of women with recurrent UTI identified frequency of sexual intercourse, use of spermi-cide, having a new sexual partner, a history of first UTI occurring before 15 years of age, and a maternal history of UTI as independent risk factors for recurrent UTI.
Pregnancy
The incidence of asymptomatic bacteriuria in pregnant women is approximately 4% to 10%, which is similar to the rate reported in sexually active nonpregnant women of childbearing age.10-12 If not treated, 20% to 40% of pregnant women with bac-teriuria in the first trimester will acquire acute pyelonephritis later in pregnancy. A recent meta-analysis estimated that treatment of asymptomatic bacteriuria would lead to approximately a 75% reduction in the incidence of pyelonephritis.12 Premature births and perinatal mortality are increased in pregnancies complicated by UTI.11,12 There is little evidence that the infections that develop during pregnancy have long-term effects.
Diabetes
The rates of asymptomatic bacteriuria and UTI in diabetic women are twofold to threefold higher than those in nondiabet-ic women; these differences have not been observed in men.13 In hospitalized diabetic patients, particularly those with multiple- organ complications, the incidence of infection and true pyelonephritis also appears to be increased, partly because of poor bladder function and urinary catheterization. Other clinical conditions causing obstruction in urinary flow or incomplete voiding also predispose diabetic patients to infection. In addition, impaired cytokine secretion may contribute to asymptomatic bacteriuria in diabetic women.
Etiology
The spectrum of organisms causing UTI varies by clinical syndrome. In acute uncomplicated cystitis, the etiologic agents are highly predictable: E. coli accounts for 75% to 90% of isolates; Staphylococcus saprophyticus accounts for 5% to 15% of isolates (particularly in younger women); and Klebsiella species, Proteus species, enterococci, and other organisms account for 5% to 10% of isolates. The spectrum of agents that cause uncomplicated pyelonephritis is less well studied than, but is similar to, that which causes acute cystitis. In complicated UTIs, E. coli remains the predominant organism, but other aerobic gram-negative rods, such as Klebsiella species, Proteus species, Citrobacter species, Acinetobacter species, Morganella species, and Pseudomonas aeruginosa are also frequently isolated. Gram-positive bacteria, such as enterococci, S. aureus, and S. epidermidis, as well as yeast, are also important pathogens in complicated UTI.
Pathogenesis
Bacteria can establish infection in the urinary tract by traveling from the urethra to the bladder and then up the ureter to the kidney. However, introduction of bacteria into the bladder does not inevitably lead to sustained infection. For example, bacteria often enter the bladder after sexual intercourse, but normal micturition and innate host defense mechanisms in the bladder eliminate these organisms. The bladder mucosal surface has antibacterial properties that eliminate some organisms, presumably through mucus trapping and a polymorphonuclear leukocyte response. In addition, urine that has a low pH, high or very low osmolarity, high urea concentration, or high organic acid content inhibits bacterial growth. Abnormal micturition, a significant residual urine volume, or both will promote true infection. There are also acquired and intrinsic host factors, as well as bacterial virulence factors, which increase the likelihood of development of UTI (see below).
Bacteria can also gain access to the urinary tract through the bloodstream. However, hematogenous spread accounts for fewer than 2% of documented UTIs and usually results from bac-teremia caused by relatively virulent organisms, such as Salmonella and S. aureus. Hematogenous infections may produce focal abscesses or areas of pyelonephritis within a kidney and result in positive urine cultures.
Vaginal ecology and uti
In women, colonization of the vaginal introitus with organisms from the fecal flora, usually E. coli, is the critical initial step in the pathogenesis of UTI. Sexual intercourse and the use of a diaphragm with spermicide or of spermicide alone are strongly associated with an increased risk of E. coli vaginal colonization and bacteriuria, probably because of alterations in the normal vaginal microflora.
In postmenopausal women, there is an increased incidence of gram-negative vaginal colonization and bacteriuria. These trends correlate with the changes in the vaginal environment that occur with menopause: disappearance of the previously predominant lactobacilli from the vaginal microflora and a rise in pH. A case-control study of community-dwelling postmeno-pausal women found significantly lower rates of vaginal colonization with lactobacilli in those women who were not taking hormone replacement therapy than in those who were using systemic or topical estrogen.16 In a randomized, placebo-controlled trial in postmenopausal women with a history of recurrent UTI, topical estrogen therapy resulted in restoration of the premenopausal vaginal flora and a decrease in both the prevalence of vaginal E. coli colonization and the incidence of UTI.
Genetic factors
There is increasing evidence that genetically determined factors may influence susceptibility to recurrent UTI. Women with recurrent UTI demonstrate a propensity for persistent vaginal colonization with E. coli, even during asymptomatic periods. Vaginal and periurethral mucosal cells from women with recurrent UTI bind threefold more uropathogenic bacteria than do mucosal cells from women without recurrent infection. These observations suggest that epithelial cells from susceptible women may possess specific types or greater numbers of receptors to which E. coli can bind, thereby facilitating colonization. This increased susceptibility is determined in part by Lewis blood group type and whether the woman secretes blood group antigens into bodily fluids. Vaginal epithelial cells from nonse-cretors of blood group antigens bind significantly greater numbers of bacteria, and nonsecretors are particularly at risk for recurrent UTI.18,19 Mutations in host-response genes (e.g., those coding for Toll receptors and the interleukin-8 receptor) have been linked to severity of UTIs in animals, although they have not yet been linked in humans.20
Bacterial virulence
Certain strains of E. coli possess chromosomally encoded virulence determinants that confer the ability to infect the anatomically normal urinary tract and produce acute inflammatory disease (e.g., cystitis and pyelonephritis). Characteristics that have been associated with uropathogenicity are the presence of certain O and K surface antigens (the O antigen is the outer polysaccharide portion of the bacterial envelope, and the K antigen is the antiphagocytic capsular antigen), the presence of the siderophore aerobactin, resistance to the bactericidal activity of serum, the ability to produce toxins such as hemolysin and cytotoxic necrotizing factor, and certain intracellular metabolic capabilities.
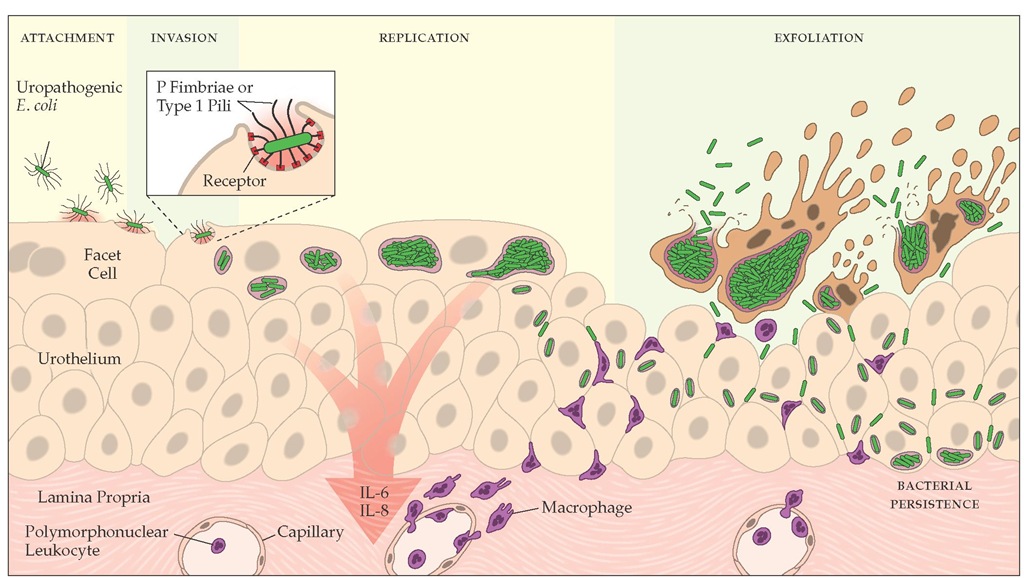
Also important is the presence of adhesins on the surface of uropathogenic bacteria that mediate binding to specific receptors on the surface of uroepithelial cells. The best-studied adhesion structure is the P fimbriae, which are hairlike protein structures found on the surface of certain pathogenic strains of E. coli. P fimbriae interact with a specific receptor on epithelial cells. This epithelial cell receptor contains the carbohydrate moiety a-D-galactopyranosyl-(1 ^ 4)-|-D-galactopyranoside, which is found in the P blood group antigens. The prevalence of P-fim-briated E. coli correlates with severity of disease the strain is likely to cause: there is a low prevalence (10% to 20%) in E. coli strains from the fecal flora of asymptomatic persons; there is a higher prevalence (50% to 60%) in strains that cause cystitis; and the highest prevalence (70% to 100%) is found in strains that cause pyelonephritis.21,22 P fimbriae also appear to be important in the pathogenesis of bloodstream invasion from the kidney. From 75% to 100% of E. coli strains isolated from the blood of otherwise healthy patients with pyelonephritis express P fimbriae. In contrast, E. coli strains that cause urosepsis in persons with compromising medical conditions are much less likely to have P fimbriae.
Figure 1 The pathophysiology of infection by uropathogenic Escherichia coli in bladder epithelial cells.20 Uropathogenic E. coli organisms attach to receptors on superficial bladder cells with P fimbriae or type 1 pili. Once contact is established, the bacteria are internalized into the cells, where they can replicate to high levels. However, attachment or invasion can result in the activation of apoptotic pathways within the cells, leading to the eventual exfoliation and clearance of infected host cells. Interactions between E. coli and the cells can also result in the induction of inflammatory cytokines, leading to the influx of polymorphonuclear leukocytes into the bladder epithelium. E. coli can escape from dying cells, thereby avoiding clearance by exfoliation, and infect surrounding and underlying epithelial cells. Within the bladder epithelium, E. coli can escape immune surveillance and persist at subclinical levels. (IL-6 — interleukin-6; IL-8 — interleukin-8)
Another adhesion structure is the type 1 pilus (fimbria), which all E. coli strains possess. Type 1 pili are also thought to play a key role in initiating E. coli bladder infection; they mediate binding to uroplakins, which are mannosylated glycoproteins on the surface of bladder uroepithelial cells.21 In addition to P fimbriae and type 1 pili, other adhesins may play a role in mediating attachment of uropathogenic strains of E. coli to the uroep-ithelium in selected circumstances.
The binding of uropathogenic E. coli to receptors on uroep-ithelial cells initiates a complex series of intracellular signaling events that alters epithelial cell function [see Figure 1].8 Chemo-kines and cytokines are synthesized and secreted, inflammatory cells are attracted into the bladder epithelium, and epithelial cells undergo apoptosis and exfoliation, carrying attached E. coli away in the urine.
Anatomic and functional abnormalities
Persons who have major anatomic and functional abnormalities of the urinary tract, including vesicoureteral reflux, ureteral obstruction, or a foreign body (e.g., a stone, a catheter, or a tumor), are markedly predisposed to UTI, particularly infections involving the kidney. In such persons, UTI can develop as a result of infection with bacteria, including E. coli strains, that are not normally uropathogenic.21 Not surprisingly, infection with nonuropathogenic strains of bacteria is also common in hospitalized patients, presumably because they have anatomic and functional abnormalities. Bacterial invasion of the prostate and incomplete bladder emptying caused by bladder outlet obstruction are the primary predisposing factors in men with UTI. Instrumentation and incomplete bladder emptying are important predisposing factors for UTI in patients with spinal cord injury or diabetes. Inhibition of ureteral peristalsis leading to vesi-coureteral reflux is important in the pathogenesis of pyelonephritis in pregnant women.
Vesicoureteral reflux plays a key role in the pathogenesis of renal infection and, more important, in the evolution of chronic renal damage. It is commonly associated with UTI in children but also occurs because of anatomic abnormalities in children without UTI. Reflux provides a direct route for infection to reach the pelvicalyceal system of the kidney; severe reflux may occur intrarenally. Renal scars caused by chronic pyelonephritis are often associated with such reflux. Most reflux-associated renal damage occurs in infancy, because with growth there is a tendency for self-correction of at least mild degrees of reflux. The long-term effects of reflux on kidney structure and function are a major justification for an aggressive radiologic approach in infants and young children with UTI [see Management, below].23
Diagnosis
Clinical presentations and laboratory findings
The clinical presentation of UTI is quite variable, ranging from asymptomatic bacteriuria to typical symptomatic cystitis to acute pyelonephritis. In addition, clinical symptoms do not always correlate with the site of infection (bladder versus kidney) or with the degree of bacteriuria. Approximately 30% of patients with lower urinary tract symptoms also have silent infection of the kidney.4 Despite considerable effort, researchers have been unable to develop a noninvasive technique for differentiating renal infections from bladder infections. The best noninvasive test to delineate the anatomic site of infection appears to be the response to short-course antibiotic therapy [see Management, below].
Cystitis
The typical symptoms of cystitis are dysuria, urinary frequency, and urgency. Nocturia and suprapubic or back discomfort are also often present. In addition, the urine may be cloudy, malodorous, or bloody.
A meta-analysis evaluating the probability of UTI on the basis of history and physical findings concluded that the probability of UTI was at least 50% in a woman presenting with one or more symptoms of UTI. If vaginal discharge and complicating factors are absent and risk factors for UTI are present, then the probability of UTI is close to 90% and no laboratory evaluation is needed. Similarly, a combination of dysuria and frequency— the most common symptoms—in the absence of vaginal discharge increases the probability of UTI to 96%. Further laboratory evaluation with dipstick or urine culture can be omitted in such patients, and empirical therapy can be considered. If the history is not clear, then a urine dipstick should be performed. A positive nitrite or leukocyte esterase result makes the probability of UTI about 80%, and empirical treatment can be considered without further testing. In this setting, a negative dipstick result does not rule out UTI, and a urine culture and close clinical follow-up are recommended. Interestingly, the only physical examination finding that increased the probability of UTI was cos-tovertebral angle tenderness (suggesting pyelonephritis), and the authors recommended that the physical examination could be omitted in patients with typical symptoms of acute uncomplicated cystitis.
On dipstick testing, the presence of leukocyte esterase, nitrite, or both has about 75% to 90% sensitivity and 70% to 82% speci-ficity.4 The pH is typically elevated, and blood may be present. Depending on the clinical circumstances, it may be appropriate to follow dipstick testing with urine culture and antimicrobial-sensitivity testing [see Management, below].
Urine microscopy reveals pyuria in nearly all cases of cystitis and hematuria in about 30% of cases. Bacteriuria is demonstrable on Gram stain of unspun urine in over 90% of cases with 105 bacteria/ml or higher. Approximately two thirds of women presenting with clinical cystitis will have urinary colony counts of 105 bacteria/ml or higher. Studies in women with symptoms of cystitis have found that a colony count threshold of greater than 102 bacteria/ml has greater sensitivity (95%) and specificity (85%) than a threshold of 105bacteria/ml for the diagnosis of acute cystitis in women4 [see Bacterial Count on Urine Culture, below].
Pyelonephritis
Patients with pyelonephritis can present with clinical manifestations that range from mild to relatively severe—from low- grade fever with lower back or costovertebral angle pain to fever, shaking chills, nausea, vomiting, and loin pain. Symptoms are generally acute in onset and may or may not be associated with symptoms of cystitis.4,25 The primary finding on physical examination is costovertebral angle tenderness on deep palpation. Tachycardia may accompany fever. Pyuria and bacteriuria are usually demonstrable on urine microscopy and Gram stain. Bacteremia may complicate the course of pyelonephritis; but in patients with pyelonephritis, bacteremia is seldom associated with the more serious sequelae of gram-negative infection (i.e., triggering of the complement, clotting, and kinin systems), which may lead to septic shock, disseminated intravascular coagulation, or both. When shock or disseminated intravascular coagulation occurs, the possibility of obstruction must be considered. In a particularly serious form of obstructive uropathy associated with acute papillary necrosis, the sloughed papillae may obstruct the ureter. This should be suspected in diabetic patients who have severe pyelonephritis and persistent bacteremia despite antibiotic therapy. Papillary necrosis may also be evident in some cases of pyelonephritis complicated by obstruction, sickle cell disease, analgesic nephropathy, or combinations of these conditions. Emphysematous pyelonephritis, which is a particularly severe form of pyelonephritis associated with production of gas in renal and perinephric tissues, occurs almost entirely in diabetic patients.
Renal and Perirenal Abscesses
Two unusual forms of UTI are macroscopic renal and perire-nal abscesses. In the past, most of these abscesses were secondary to hematogenous infection with S. aureus. Currently, most of them are secondary to ascending UTI with the usual En-terobacteriaceae organisms. Such infections are often complicated by renal calculi and obstruction of urinary flow from either the kidney or the ureter. Less commonly, preexisting renal cysts become infected and develop into abscesses. In rare instances, there is contiguous spread from a neighboring site of suppuration, such as the colon or overlying rib. The usual presentation of such infections is insidious, with chronic fever, weight loss, night sweats, and anorexia, and is often associated with flank or back pain. When the abscess is under pressure, usually because of obstruction, a more acute presentation with associated bac-teremia may occur. Symptoms specific to the urinary tract, such as dysuria, hematuria, and urinary retention, are sometimes noted but are often absent. On physical examination, costovertebral angle tenderness or even a palpable mass may be found; in 30% to 50% of patients, however, this finding is absent.
Routine laboratory tests are of variable value in patients with renal or perirenal abscesses: leukocytosis may be present, anemia is not unusual, and signs of inflammation (e.g., pyuria or proteinuria) may be evident on urinalysis. In more than half of patients with renal or perirenal abscesses, the organism in the abscess may be isolated on urine culture. Definitive diagnosis, however, depends on radiographic detection of a mass lesion. Gallium and ultrasound scans may be helpful, but a computed tomographic or magnetic resonance imaging scan is considered the diagnostic test of choice.26 If prompt drainage and antibiotic therapy are not provided, renal or perirenal abscesses may extend to the peritoneal cavity, chest, or skin.
Prostatitis
A common complication of UTI in men is prostatitis. Bacterial prostatitis is usually caused by the same gram-negative bacilli that cause UTI in females; 80% or more of such infections are caused by E. coli. The pathogenesis of this condition is poorly understood. Antibacterial substances in prostatic secretions probably protect against such infections.
A National Institutes of Health expert consensus panel has recommended classifying prostatitis into three syndromes: acute bacterial prostatitis, chronic bacterial prostatitis, and chronic pelvic pain syndrome (CPPS). Acute bacterial prostatitis is a febrile illness characterized by chills; dysuria; urinary frequency and urgency; and perineal, back, or pelvic pain. Bladder outlet obstruction may occur. On physical examination, the prostate is found to be enlarged, tender, and indurated. Pyuria is present, and urine cultures generally grow E. coli or another typical uropathogen.
Chronic bacterial prostatitis is a clinically more occult disease and may be manifested only as recurrent bacteriuria or variable low-grade fever with back or pelvic discomfort. Urinary symptoms usually relate to the reintroduction of infection into the bladder, with both pyuria and bacteriuria being present; a chronic prostatic focus is the most common cause of recurrent UTI in men.
CPPS describes the large group of men who present with minimal signs on physical examination but have a variety of irritative or obstructive voiding symptoms; perineal, pelvic, or back pain; and sexual dysfunction. These men can be divided into those with and those without inflammation (defined as > 10 white blood cells per high-power field in expressed prostatic secretions). The etiology and appropriate management in these patients, regardless of inflammatory status, is unknown.