Evaluation and acute management
Although the emergency department evaluation and management of MTBI is controversial, the principal concern is with identifying evolving surgical lesions such as hematomas and contusions. The cause of injury can be a factor in management; for example, motor vehicle accidents involving large forces or impact to the head may raise the likelihood of hematomas, as do falls in older patients, especially if the patient is taking anticoagulants or aspirin. Documentation of the history of the injury, as well as the length of the period of unconsciousness, mental confusion, or both, can be very helpful in both acute and long-term management. In the past, many patients with traumatic loss of consciousness were admitted to the hospital for overnight observation, especially if there was no responsible adult to be with the patient at home. However, changes in managed care over the past several decades and the increased availability of computed tomography have led to a marked decrease in such admissions.
In addition to history and examination, CT has become the mainstay of evaluation, to the exclusion of skull x-ray. Prolonged or deteriorating mental status or the presence of neurologic signs or other risk factors are still clear indications for CT scanning, observation, or both after MTBI. For example, MTBI patients whose GCS scores are 13 or 14 on admission have a much higher incidence (up to 28%) of abnormal findings on CT scanning than do patients with a GCS score of 15.43 In addition, on rare occasions, even MTBI patients whose initial CT scans are negative may develop surgical complications after discharge; repeat CT scanning should thus be considered in patients who return with severe, persistent symptoms or new neurologic signs.
The diagnosis of contusion is also important for its longer-term prognostic value. Patients with MTBI complicated by cerebral contusion have a 6-month outcome that is more consistent with that of patients with moderate head injury39 and are thus candidates for more intensive, longer-term observation and management.
In the postacute period, MRI, single-photon emission computed tomography (SPECT), and quantitative EEG can provide additional documentation and localization of brain injury that can be very valuable in guiding nonsurgical management [see Figure 1]. For example, MRI can help provide a presumptive diagnosis of diffuse axonal injury and subtle brain contusions that might have been be missed on CT. Standard neuropsychological testing is usually not indicated in the MTBI patient, but some brief, specialized cognitive batteries that have been developed for this population can be very helpful in diagnosis and follow-up. These include the Sideline Assessment of Concussion (SAC) and the Automated Neuropsychological Assessment Metric (ANAM).2829,4446
Management of mtbi
Postconcussive symptoms, which include headache, dizziness, fatigue, and documented deficits in cognition, can be seen even after mild injuries without loss of consciousness. In general, the prognosis for recovery is very good, with most cognitive and somatic sequelae improving markedly by 3 months; 85% of patients experience no disabling symptoms 1 year after injury.40,47 In the small percentage of patients who have postconcussive complaints and disability over periods exceeding 1 year, psycho-genic factors can often contribute to the persistence of symptoms. Patients who have persistent symptoms of anxiety, depression, or both need appropriate diagnosis and treatment, preferably by a psychiatrist who has experience with TBI.
Probably the most important element in the longer-term management of patients with MTBI is the clinician’s recognition that the postconcussive symptoms in these patients have a structural cause and that the patients usually recover. Attention deficits and fatigue appear to be especially common and troubling, and there is nothing more disconcerting to the intelligent but symptomatic MTBI patient than to be told by his physician, family, or employer that there is nothing wrong. Early symptomatic management and counseling as to what to expect can help avoid the all too common delayed emotional symptoms of anxiety and depression, especially in patients who may be at risk because of underlying psychopathology.
Moderate and Severe Head Injury
Initial Evaluation and Resuscitation
An organized team approach to the acute management of the unconscious patient with TBI is essential and includes prehospi-tal, intensive care unit, and post-ICU care. A cornerstone of early evaluation and care is recognition of deterioration in patient status through the sequential use of a standardized measure such as the GCS score [see Figure 2], along with checks of lateralized deficits in neurologic function and careful attention to pupillary responses. The history should be obtained from witnesses, particularly with regard to the onset of coma. For example, if a patient who is comatose had an initial interval of lucidity or semi-lucidity, an expanding mass lesion may be present, and severe diffuse axonal injury is less likely.
The importance of cardiopulmonary resuscitation in patients with acute TBI cannot be overstated.50 Airway and shock management should be the first priority in all trauma patients. The loss of cerebrovascular autoregulation places the brain at increased risk for cerebral ischemia from systemic hypotension, and levels of hypercapnia tolerated by the normal brain can lead to critical marginal increases in intracranial pressure (ICP) in the patient with head injury. Most prehospital deaths after TBI are probably caused by vascular and respiratory failure. This is supported by the marked improvements in outcome achieved by emergency care systems with early prehospital intubation and resuscitation.
Shock is usually caused by hemorrhage elsewhere in the body, not in the head. Cerebral perfusion pressure should be maintained above 70 mm Hg by vigorous management of hypotension. Fluid resuscitation with normal saline or lactated Ringer solution is generally recommended, but patients with TBI should not receive excessive hydration, and central venous pressure should be monitored. Glucose administration should be avoided because it has been linked to poor outcome, possibly through increased lactic acidosis.
Comatose patients with TBI are often hypoxic or hypercapnic, even though ventilation may appear to be normal. Patients who are in a coma (i.e., those with a GCS score < 8) should undergo gentle hyperventilation, via intubation if necessary, until a carbon dioxide tension (PCO2) of about 35 mm Hg is achieved. Short-term hyperventilation to levels of about 25 mm Hg can be lifesav-ing in the patient with impending tentorial herniation. However, the recommended standard is that chronic hyperventilation be maintained at a PCO2 no lower than 25 mm Hg, because lower levels reduce cerebral blood flow and have a negative impact on outcome. Sedation or pharmacologic paralysis should be used when necessary to control acute agitation. The head should be elevated and immobilized in the plane of the body for airway maintenance and facilitation of cranial venous return.
Finally, the special nutritional requirements of the TBI patient also need particular attention, from coma through subacute recovery. Early nutritional support (often parenteral) may be associated with improved survival and decreased disability.
Radiologic Examination
CT has revolutionized the diagnosis and management of mass lesions in patients with head trauma; it should be performed in all patients with a GCS score of less than 15 and in those who have focal signs or posttraumatic amnesia. Comatose patients must be accompanied by trained personnel on the way to the CT suite because patients who are assumed to be stabilized may suffer respiratory arrest or irreversible brain damage as a result of simple airway problems en route.
The principal role of CT is in the diagnosis and management of acute surgical lesions. Hemorrhage can occur in the subarach-noid, subdural, epidural, and intraventricular spaces or in the brain parenchyma. Subdural and epidural hematomas should be evacuated promptly when associated with a significant mass effect, because it has been shown that there is a significantly poorer outcome with surgical delays of greater than 4 hours.53,54 However, surgical management of intraparenchymal hemorrhage will vary, depending on the size, mass effect, location, and neurologic status. Intraventricular hemorrhage will generally require ventricular drainage, and it has also been associated with a worse prognosis. Obliteration of the basilar cisterns from mass effect also portends a worse outcome, as does diffuse hypodensi-ty typical of cerebral hypoxia.
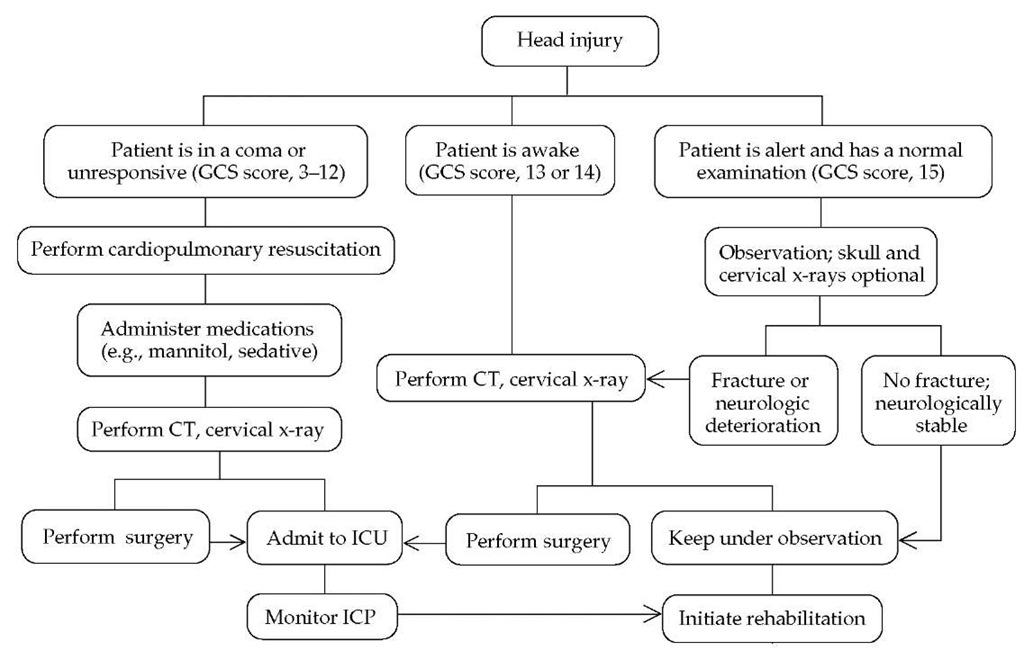
Figure 2 Management algorithm for patients with traumatic brain injury. (GCS—Glasgow Coma Scale; ICP—intracranial pressure)
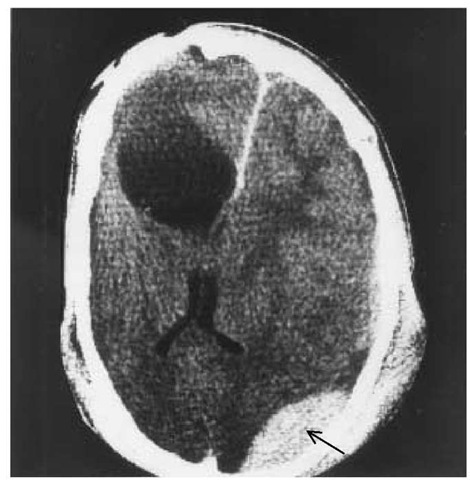
Figure 3 CT in a 19-year-old man with a history of head injury and recent occipital injury shows the old lesion, the resulting midline distortion, and an epidural hematoma (arrow) resulting from the recent injury.
CT has made the skull x-ray all but obsolete, and the latter rarely affects management.55 Normal findings on initial skull x-ray or CT should not lull the clinician into ignoring the possibility that a delayed hematoma may develop, although this is fortunately relatively rare [see Figure 3]. Whether in the hospital or at home with a responsible adult, observation remains a critical element of care, even for patients with MTBI.
MRI promises to be very useful in the long-term management of moderate and severe TBI as well as in the documentation of brain pathology in patients with milder injury. However, it is often impractical and not cost-effective in the acutely comatose patient and is not as good as CT for diagnosis of acute hematomas.
Laboratory tests should include a complete blood count; measurements of electrolytes, glucose, arterial blood gases, and blood alcohol; liver and kidney function tests; and a toxicology screen. In addition, because coagulopathies frequently occur after TBI, other tests may be indicated. Such tests include a platelet count; prothrombin, partial thromboplastin, and thrombin times; and an evaluation of fibrinogen and fibrinogen degradation products.
Acute Management
Acute management of severe head trauma is aimed at minimizing the progression or the effects of secondary injury. Although there are no specific treatments targeting the biochemical events discussed (see above), much progress has been made in early resuscitation and the management of elevated ICP. Evidence-based guidelines and standards for management of patients with severe acute TBI, as well as options for the care of such patients, have been updated by the Joint Section on Neuro-trauma and Critical Care and the American Association of Neurological Surgeons, under sponsorship of the Brain Trauma Foundation.56 These guidelines are also available through the Brain Trauma Foundation Web site (www.braintrauma.org). They represent a major advance in standardization of the management of severe TBI and should be consulted by anyone involved in the care of these patients.
Aspects of care described in the guidelines include early resuscitation; ICP monitoring; ICP treatment threshold and methods; and the use of mannitol, barbiturates, nutrition, hyperventi-lation, corticosteroids, and prophylactic anticonvulsants. It is important to note that even guidelines that are presented as options for care are very valuable, because such options represent the consensus of experts in areas where studies documenting more definitive levels of certainty are not available or are not possible [see Table 3]. The guidelines currently support a standard recommendation against the routine use of aggressive hyperventila-tion, corticosteroids, and prophylactic anticonvulsants.
The Intensive Care Unit, Intracranial Pressure
Monitoring, and Cerebral Perfusion Pressure
After a mass lesion has been surgically treated or excluded, the comatose patient should be managed in the ICU. Preventing secondary insults to the brain remains the principal goal of therapy. In general, the same principles of care that are applied in earlier stages of treatment (see above) are applied at this stage, but better monitoring is available in the ICU. Organization, training, and adherence to relatively simple principles are the mainstay of care.
ICP and cerebral perfusion pressure (CPP) are probably the most sensitive measures for monitoring the patient with severe TBI, and the results of these assessments correlate significantly with outcome.57-59 The current guideline recommends that ICP be monitored in comatose patients in whom CT yields abnormal results and in those in whom CT is normal but who have two or more of the following risk factors: age greater than 40 years, motor posturing, or a systolic blood pressure of less than 90 mm Hg. The particular monitoring technique to be used is determined by the neurosurgeon and the facilities available. An intraventricular catheter is recommended, because it can also be used for ventricular drainage as needed, though fiberoptic epidural or subdural transducers can also be used.60
The current standard measures for control of elevated ICP include sedation, paralysis, controlled hyperventilation, use of mannitol and other osmotics, ventricular drainage, and barbiturate coma [see Table 4]; these interventions are usually undertaken in that sequence to maintain an ICP of lower than 20 mm Hg.58,61 It is recommended as a guideline that mannitol be given in intermittent boluses of 0.25 to 1.0 g/kg every 4 hours as needed, but serum osmolarity should be kept below 320 mOsm/L because of concerns about renal failure. Use of a Foley catheter is strongly recommended to monitor urine output and help maintain euvolemia through adequate fluid replacement. Barbiturate coma significantly improves outcome in patients younger than 45 years with otherwise uncontrolled ICP. This is the last step recommended as a guideline in the nonsurgical control of ICP.
Barbiturate coma is induced with pentobarbital at an initial loading dose of 10 mg/kg I.V. over 30 minutes, and serum levels should then be maintained at 3 to 4 mg/dl with dosages of about 1 mg/kg/hr. The literature supports a standard recommendation that corticosteroids not be used for neuroprotection or control of ICP in patients with severe TBI.62 Finally, progressive elevations in ICP may be caused by lesions that require surgery, such as delayed hematoma or hydrocephalus. Similarly, seizures, hyponatremia, and airway problems will raise ICP.
Management of agitation
Patients with TBI often experience agitation during the immediate recovery period. Nonpharmacologic interventions, including limiting environmental stimuli and providing gentle interaction with the patient, are of great importance in early management and should typically be the first line of therapy. When ICU patients require chemical restraint for their own safety and the safety of staff, propofol may be given intravenously to manage agitation; the recovery time with propofol is quicker than with benzodiazepines such as midazolam.
After the patient has left the ICU, benzodiazepines (e.g., lo-razepam, 0.5 to 2.0 mg p.o. or I.M., or clonazepam, 0.5 to 2.0 mg p.o.) may be the first choice, either alone or in combination with carbamazepine or valproate. However, benzodiazepines may cause disinhibition in some patients with brain injury. Neurolep-tics (e.g., molindone, 10 mg p.o., b.i.d., or haloperidol, 0.5 to 2.5 mg p.o., b.i.d.) are less desirable because they may cause ex-trapyramidal effects, akathisia (a subjective sense of restlessness that may prolong agitation), or both. Some newer agents, such as olanzapine, 2.5 to 5.0 mg/day orally, may have fewer side ef-fects. Animal models suggest that neuroleptics have a negative long-term effect on recovery.63 Dopamine agonists such as aman-tadine and bromocriptine have also been successfully used for postcoma agitation caused by impairment of dopaminergic and other ascending monoaminergic pathways.
Table 3 Evidence-Based Guidelines for the Management of Severe Head Injury62
|
Subject |
Certainty Level* |
Recommendations |
|
Trauma systems |
Guideline |
All regions in the United States should have an organized trauma care system |
|
Resuscitation |
Guideline Option |
Systolic BP < 90 mm Hg or hypoxia must be scrupulously avoided or corrected immediately Mean arterial pressure should be kept above 90 mm Hg |
|
Integration of brain-specific treatments into initial resuscitation |
Option |
When clear signs of transtentorial herniation are present, the herniation should be treated aggressively; hyperventilation should be performed rapidly (mannitol is desirable with adequate volume resuscitation); sedation and short-acting neuromuscular blockade can be used but may interfere with the neurologic examination |
|
ICP monitoring |
Guideline |
Admission GCS 3-8 plus abnormal CT scan, or GCS 3-8 and normal CT plus age > 40 years, or motor posturing, or systolic BP < 90 mm Hg |
|
ICP treatment threshold |
Guideline Option |
ICP > 20-25 mm Hg ICP treatment should be corroborated by frequent clinical examination and cerebral perfusion pressure data |
|
ICP treatment critical pathway |
Option |
[See text, Table 4, and reference 47] |
|
CPP |
Option |
Maintain CPP above 70 mm Hg |
|
Hyperventilation |
Standard Guideline Option |
In the absence of increased ICP, chronic prolonged hyperventilation (Paco2 < 25 mm Hg) should be avoided Prophylactic hyperventilation (Paco2 < 35 mm Hg) should be avoided during the first 24 hr after severe TBI because it can compromise CPP when cerebral blood flow is reduced Hyperventilation therapy may be necessary for brief periods when there is neurologic deterioration or when ICP elevations are refractory to other treatment |
|
Mannitol |
Guideline Options |
Mannitol is effective for ICP control; intermittent boluses (0.25 to 1.0 g/kg) may be more effective than continuous infusion Indications for mannitol before ICP monitoring are progressive neurologic deterioration or transtentorial herniation not attributable to systemic pathology Maintain serum osmolality < 320 mOsm Maintain euvolemia by adequate fluid replacement; use Foley catheter |
|
Barbiturates |
Guideline |
High-dose barbiturates may be used in hemodynamically stable severe TBI patients with ICP elevations refractory to maximal medical and surgical therapy |
|
Glucocorticoids |
Standard |
Glucocorticoids are not recommended for ICP control or improving outcome in severe TBI patients |
|
Nutritional support |
Guideline Option |
Replace 140% of resting metabolic expenditure (100% in paralyzed patients) by using enteral or parenteral formulas, with at least 15% of calories as protein Feeding by gastrojejunostomy is preferred |
|
Antiseizure prophylaxis |
Standard Option |
Prophylactic use of phenytoin, carbamazepine, or phenobarbital is not recommended for preventing late posttraumatic seizures Short-term (1 wk) phenytoin or carbamazepine is recommended to prevent early posttraumat-ic seizures in high-risk patients after head injury |
Note: This table should not be used alone as a guide to therapy. Clinicians are referred to the full guideline document (see text).
*For determining certainty level, studies that are controlled and randomized rank highest, and expert opinion ranks lowest. The highest level of certainty is represented by standards; the next highest, by guidelines; and the lowest, by options for care.
BP—blood pressure
CPP—cerebral perfusion pressure
GCS—Glasgow Coma Scale
ICP—intracranial pressure
Paco2—arterial carbon dioxide tension
TBI—traumatic brain injury
Table 4 Management of Intracranial Pressure
|
Treatment |
Dosage |
|
Sedation (with morphine) |
As needed |
|
Paralysis (with pancuronium) |
As needed |
|
Ventricular drainage |
As needed |
|
Mannitol |
0.25-1.0 g/kg q. 4 hr |
|
Hyperventilation |
To a Pco2 of 35 mm Hg (25 mm Hg only for brief periods, if needed for transtentorial herniation) |
|
Barbiturate coma (pentobarbital) |
Loading dose of 10 mg/kg, then 1 mg/kg/hr |
Pco2—carbon dioxide tension
A recent survey of rehabilitation physicians suggests that those who are more experienced in caring for patients with brain injury tend to use carbamazepine and beta blockers in preference to neuroleptics for management of agitation. In all cases of prolonged confusion or agitation, however, other causes must also be considered, such as the side effects of medication, infection, electrolyte imbalance, hypoxia, and late in-tracranial complications.