Prone Positioning
An effective method for improving oxygenation is to place the patient in the prone position.21,22 The mechanism by which the prone position improves oxygenation is related to the reduction of shunting and correction of V/Q mismatching. The recruitment of dorsal atelectatic lung is thought to be produced by a more even distribution of pleural pressure. The transmural distending pressure is greater in the dorsal regions but is not significantly reduced in the dependent ventral region. Studies have found that in 60% to 80% of ARDS patients who are placed in the prone position, the Pao2-Fp2 ratio improves (from 80 to 200).22 However, it is not clear whether prone positioning alters mortality for ARDS patients.
Complications of Mechanical Ventilation
Pulmonary complications
Serious pulmonary complications of intubation and mechanical ventilation can be divided into three categories: (1) infection related to the presence of an endotracheal tube, (2) alveolar overdistention, and (3) atelectasis.
Infection
Mechanically ventilated patients are at high risk (13% to 38%) for nosocomial pneumonia.23 Early-onset pneumonia, occurring 48 to 72 hours after intubation, is usually the result of aspiration during the intubation process.24 These infections are most often caused by antibiotic-sensitive organisms, including oxacillin-sensitive Staphylococcus aureus, Haemophilus influenzae, and Streptococcus pneumoniae. Ventilator-associated pneumonia that occurs more than 72 hours after intubation is frequently caused by antibiotic-resistant pathogens, including Pseudomonas aeruginosa, oxacillin-resistant S. aureus, Acinetobacter species, and Enterobac-ter species. The pathogenesis of ventilator-associated pneumonia usually requires two important steps: (1) bacterial colonization of the aerodigestive tract and (2) aspiration of contaminated secretions into the lower airways. The risk of a ventilator-associated pneumonia appears to be higher in trauma or burn patients, and in all patients, the risk rises as the duration of ventilation increases.23,25 Diagnosis of ventilator-associated pneumonia may be difficult because many other processes may cause pulmonary infiltrates and fever. Also, cultures obtained by suction-ing secretions through the endotracheal tube do not reliably differentiate between pneumonia and bacterial colonization of the trachea. The use of the fiberoptic bronchoscope to obtain specimens with a protected brush or quantitative cultures of bronchial lavage fluid may be helpful in excluding a pulmonary source of infection in intubated patients who have new clinical signs that may be caused by a noninfectious process.
Nonpharmacologic strategies may decrease the incidence of ventilator-associated pneumonia, including adequate hand washing after contact with each patient. Whenever possible, in-tubated patients should be kept in a semirecumbent position (45° from horizontal), and gastric distention should be avoided to prevent aspiration.27 In addition, the incidence of ventilator-associated pneumonia can be significantly reduced with the continuous aspiration of subglottic secretions.28 Several pharma-cologic strategies may prevent the development of ventilator-associated pneumonia, such as avoiding the use of unnecessary antibiotics, rotating the class of antibiotics used in the empirical treatment of a suspected bacterial infection, and administering chlorhexidine oral rinse.29
Nosocomial sinusitis is strongly related to the nasotracheal route of intubation. In one study, CT demonstrated fluid in the maxillary sinus in 95.5% of patients who underwent both naso-tracheal and nasogastric intubation for 1 week, compared with only 22.5% of patients in whom endotracheal and feeding tubes were placed via the oral route.30 Aspiration of the sinus may reveal nonpurulent mucoid material, but in a significant number of cases, sinusitis will be evidenced by positive stains and cultures of aspirated pus. Treatment of nosocomial sinusitis includes administration of antibiotics, replacement of nasal tubes with oral tubes, and use of decongestants to facilitate drainage.31
Alveolar Overdistention
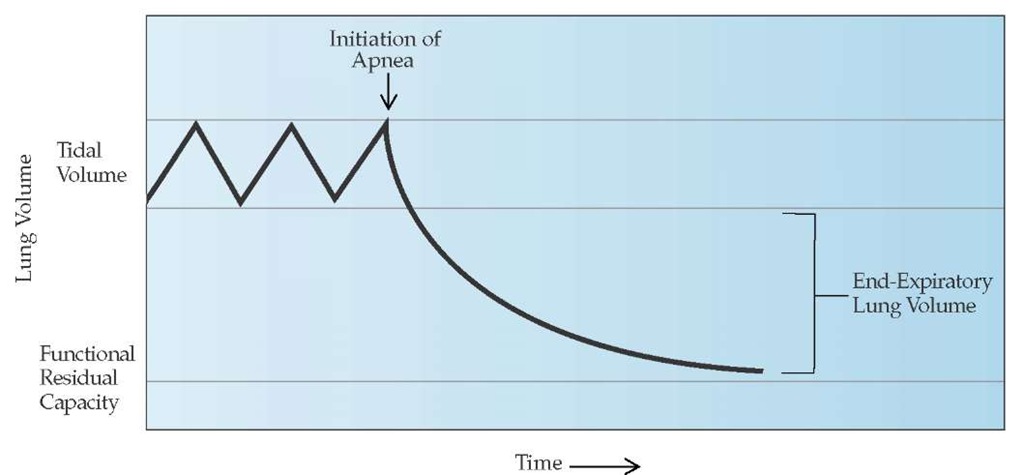
Alveolar overdistention results in two potentially life-threatening problems: hypotension and barotrauma. Ventilator-associated hypotension occurs most often in patients with obstructive airway disease, because the markedly increased lung volume with auto-PEEP impedes venous return. The risk of life-threatening hypotension is greatest at the time of intubation, when preexisting volume depletion and the administration of sedative agents limit the patient’s ability to maintain blood pressure. In addition to receiving rapid infusion of intravenous fluids, hypotensive patients who have underlying airway disease should be allowed to reduce the overdistention themselves by interrupting mechanical inflation, before ventilation is resumed at a reduced frequency [see Figure 5]. Although less common, ventilator-associated hypotension also occurs in ARDS patients who have high levels of PEEP and when intrinsic PEEP develops with airway obstruction.
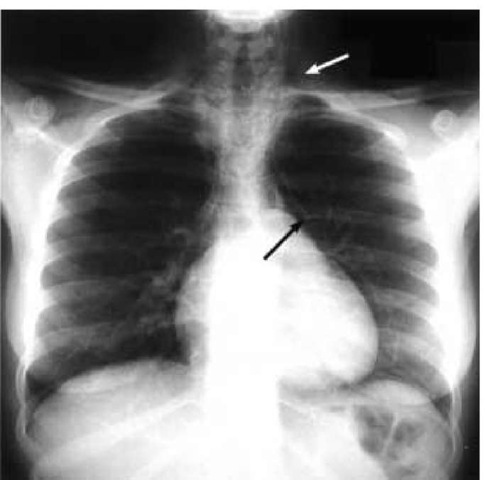
Extra-alveolar air caused by positive pressure ventilation is termed barotrauma. Examples include subcutaneous emphysema, pneumomediastinum, pulmonary interstitial emphysema, pneumoperitoneum, arterial gas embolism, and pneumothorax [see Figure 6]. Extra-alveolar air usually originates from overdis-tended alveoli that rupture into the surrounding interstitial space. High alveolar (plateau) pressure and infections that produce lung necrosis increase the risk of barotrauma. Tension pneumothorax is the most common life-threatening manifestation of barotrauma. Tension pneumothorax leads to worsening hypoxemia and decreased venous return with hypotension. Hy-perresonance and a reduction of breath sounds on the side of the pneumothorax are common, and inflation pressures are increased. In patients with severe airflow obstruction, differentiat-ing a tension pneumothorax from hyperinflation as the cause of hypotension may be difficult at bedside. Therefore, it is advisable to allow a period for deflation to determine whether a reduction of hyperinflation improves the blood and inflation pressure [see Figure 5].
Figure 5 The degree of pulmonary hyperinflation above the functional residual capacity can be determined by measuring the total exhaled volume during a period of apnea of 20 to 40 seconds. This allows the patient to reach the passive relaxation volume of the respiratory system. The difference between the total exhaled volume and the tidal volume represents the amount of pulmonary hyperinflation or the end-expiratory lung volume.
Figure 6 Chest radiograph displays several signs of barotrauma, including subcutaneous emphysema (white arrow) and pneumo-mediastinum (black arrow).
Atelectasis
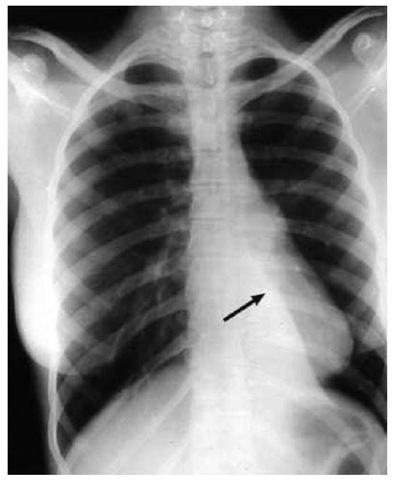
Atelectasis is a common cause of severe hypoxemia that develops during mechanical ventilation. Left lung atelectasis may result from intubation of the right mainstem bronchus, a problem that also may lead to overdistention of the right lung signaled by increased inflation pressures [see Figure 7]. A common cause of atelectasis is mucoid impaction of the bronchi. The right lung is more easily suctioned because the right mainstem bronchus follows a more direct course. The left lung is more likely to be affected by retained secretions because of the more horizontal course of the left mainstem bronchus. With atelectasis of an entire lung, breath sounds are diminished or absent on the affected side, and the trachea is shifted toward that side. A chest radiograph will reveal increased opacity in the affected hemi-thorax, together with ipsilateral tracheal shift and elevation of the hemidiaphragm. These findings are crucial for radiographic differentiation of whole lung atelectasis from a massive pleural effusion. Massive pleural effusion should cause the trachea to deviate away from the involved lung. A large region of atelectasis may produce a significant intrapulmonary shunt, giving rise to profound hypoxemia caused by shunting that is refractory to an increase in FjO2.
Atelectasis should be suspected when a sudden onset of severe, refractory hypoxemia occurs in the absence of hemody-namic instability. Other causes of profound hypoxemia, such as massive pulmonary embolism or tension pneumothorax, produce concomitant hypotension. Placing the patient in the lateral decubitus position with the atelectatic lung superior may significantly improve oxygenation, because gravity will redistribute blood flow to the dependent lung. Bronchoscopy should be performed to remove excess mucus if it cannot be easily extracted with endotracheal suctioning after chest percussion.
Approach to Pulmonary Complications
Worsening respiratory distress or arterial oxygen desaturation may develop suddenly as a result of changes in the patient’s cardiopulmonary status or secondary to a mechanical malfunction. The first priority is to ensure patency and correct positioning of the patient’s airway so that adequate oxygenation and ventilation can be administered during the ensuing evaluation. Briefly, note ventilator alarms, airway pressures, and tidal volume. Low-pressure alarms with decreased exhaled tidal volumes may suggest a leak in the ventilator circuit. Disconnect the patient from the ventilator and manually ventilate with an anesthesia bag, using 100% oxygen. For patients receiving PEEP, manual ventilation with a PEEP valve should be used to prevent atelectasis and hypoxemia. If manual ventilation is difficult, check airway patency by passing a suction catheter through the endotracheal tube or tracheostomy tube. Additionally, listen for prolonged expiration continuing up to the point of the next manual breath. This suggests the presence of gas trapping and auto-PEEP. Check vital signs and perform a rapid physical examination, with attention paid to the patient’s cardiopulmonary status. Be attentive to asymmetry in breath sounds or tracheal deviation suggesting tension pneumothorax. Note other parameters, including cardiac rhythm and hemodynamics. Treat appropriately on the basis of the foregoing evaluation.
Treatment should be specific to the identified problems. If the presence of gas trapping and auto-PEEP is suspected, a reduction in the minute ventilation is appropriate. In some circumstances, periods of hypoventilation (4 to 6 breaths/min) or even apnea for 30 to 60 seconds may be necessary to reverse the he-modynamic sequelae of auto-PEEP (e.g., shock and electromechanical dissociation). Return the patient to the ventilator only after checking its function. Increase the level of support provided by the ventilator to the patient after an episode of respiratory distress or arterial oxygen desaturation. Usually, this adjustment means increasing the FjO2 and the delivered minute ventilation unless significant auto-PEEP is present. An acute increase in the peak airway pressure usually implies either a decrease in lung compliance or an increase in airway resistance. At a minimum, considerations that should be entertained as causes of increased airway pressure include (1) pneumothorax, hemothorax, or hydropneumothorax; (2) occlusion of the patient’s airway; (3) bronchospasm; (4) increased accumulation of condensate in the ventilator circuit tubing; (5) mainstem intubation; (6) worsening pulmonary edema; or (7) the development of gas trapping with auto-PEEP. Loss of tidal volume, as evidenced by a difference between the tidal volume setting and the delivered tidal volume, implies a leak in either the ventilator or the inspiratory limb of the circuit tubing. A difference between the delivered tidal volume and the expired tidal volume implies the presence of a leak at the patient’s airway, from cuff malfunction, malpositioning of the airway (e.g., positioning of the cuff at or above the level of the glottis), or a leak within the patient (e.g., a bronchopleural fistula in a patient with a chest tube).
Asynchronous breathing (so-called fighting or bucking the ventilator) occurs when a patient’s breathing coordinates poorly with the ventilator. This difficulty may indicate unmet respiratory demands. A careful evaluation is mandated, with attention focused at the identification of leaks in the ventilator system or airway, inadequate FIO2, or inadequate ventilatory support. The problem can be alleviated by adjustments in the mode of mechanical ventilation, rate, tidal volume, inspiratory flow rate, and level of PEEP. The identification of gas trapping with auto-PEEP may require changing multiple settings to allow adequate time for exhalation (e.g., decreasing rate and tidal volume, increasing inspiratory flow rate, or switching from assist-control to SIMV in selected cases). Measures aimed at reducing the work of breathing with mechanical ventilation also may resolve the problem (addition of flow-by triggering or low levels of PSV to patients taking spontaneous breaths). If these adjustments are unsuccessful, sedation should be attempted. Muscle paralysis should be reserved for patients in whom effective gas exchange and ventilation cannot be achieved with other measures.
Nonpulmonary complications
Nonpulmonary complications occur in critically ill patients either because of the natural course of the underlying disease or because of iatrogenesis. A major role of the physician is to limit the occurrences of these nonpulmonary complications by anticipating common problems and initiating specific prophylactic measures.
Venous Thromboembolism
Venous thromboembolic disease is a significant cause of morbidity and mortality in critically ill patients. On routine clinical screening, 33% of patients in medical intensive care units have deep vein thrombosis (DVT) and 18% of trauma patients have proximal DVT.33,34 Specific independent risk factors associated with the development of venous thromboembolism include trauma, underlying malignancy, immobilization, heart failure, and obesity. Prophylaxis is recommended for all high-risk patients and has been reported to decrease the incidence of DVT by 68%.35 The most commonly used regimen is low-dose unfrac-tionated heparin, 5,000 units administered subcutaneously two or three times daily. Other prophylactic therapies, including intermittent pneumatic compression stockings and graded elastic stockings, can be used for patients who cannot tolerate anticoag-ulation. In patients with major trauma, low-molecular-weight heparin is more effective in preventing the development of proximal DVT than is prophylaxis with standard unfractionated heparin.36 In medical patients, however, the use of low-molecular-weight heparin as prophylaxis has not been shown to provide greater benefit than standard unfractionated heparin.37
Figure 7 Chest radiograph shows a collapsed left lower lobe of the lung (arrow).
Gastrointestinal Bleeding
Gastrointestinal bleeding caused by stress ulceration is another important nonpulmonary complication and occurs in 1% to 10% of all critically ill patients.38 Patients with coagulopathy, burns, head injury, or respiratory failure requiring mechanical ventilation are at increased risk for clinically significant bleeding. H2 receptor antagonists should be administered to high-risk patients to reduce the possibility of the development of clinically significant bleeding.39 Other therapeutic options include the cytoprotective agent sucralfate, which does not alter the gastric pH level and may be associated with a lower risk of late-onset pneumonia.40
Pressure Ulcers
Critically ill patients are also at an increased risk for pressure ulcers or localized areas of tissue necrosis that develop when soft tissue is compressed between a bony prominence and an external surface.41 Pressure ulcers occur in 33% to 56% of patients in the ICU and are a source of infection that can result in bac-teremia and osteomyelitis. Prevention programs that include regular repositioning of patients, which reduces the accumulation of moisture on skin, and adequate nutritional supplementation decrease the incidence of pressure sores. Air-suspension beds that redistribute body weight away from bony prominences also reduce the risk of pressure sores in certain critically ill patients.42
Neuromuscular Weakness
Patients who undergo mechanical ventilation may be at risk for neuromuscular weakness that persists long after the cause of respiratory failure has been resolved. A common cause of diffuse weakness is critical illness polyneuropathy, an axonal disorder that occurs with sepsis and multiorgan failure.43 When present, critical illness polyneuropathy may be an important cause of delayed weaning from mechanical ventilation. Use of neuromuscular paralysis may be associated with weakness that persists after the neuromuscular blocking agents have been discontinued. Prolonged neuromuscular blockade can be diminished by appropriate dosing, adequate monitoring of the degree of neuromuscular blockade, and avoidance of medications that potentiate the action of specific neuromuscular blocking agents.44 Neuromuscular blocking agents may also contribute to the development of acute myopathy, particularly in patients who receive concomitant corticosteroids. The risk of myopathy is not influenced by the chemical structure of the agent that is used to induce paralysis but, rather, is strongly correlated with the duration of paralysis.
Acute Renal Failure
Acute renal failure is a common nonpulmonary complication of patients in the ICU. Despite considerable advances in the management of critically ill patients and renal replacement therapy, the mortality associated with acute renal failure remains greater than 50%. Multiple system organ failure and other co-morbidities contribute to the high mortality associated with acute renal failure, but the acute renal failure is also independently associated with an increase in morbidity and mortality.45,46 In addition, long-term dialysis support is sometimes required for survivors of acute renal failure. Renal dysfunction in ICU patients is usually caused by intrinsic renal disease.47 Hypotension, sepsis, the use of aminoglycosides, and volume depletion are all risk factors for the development of acute renal failure.
Withdrawal of Mechanical Ventilatory Support
To decrease complications and improve patient comfort, mechanical ventilatory support should be removed as soon as possible. However, premature withdrawal of mechanical ventilation is also associated with adverse events that may further delay appropriate extubation, such as aspiration or severe cardio-pulmonary decompensation. Determination of the proper time to extubate a patient is based on several factors, including reversal or improvement of the underlying acute illness, adequate respiratory function, lack of excessive secretions, and ability to protect the airway.
Simple screening criteria have been developed that identify patients who may be ready to be removed from mechanical ventilation. These criteria include a minimal requirement for supplemental oxygenation (Fp2 < 0.40 or PaO2/Fp2 < 200), a PEEP level not exceeding 5 cm H2O, adequate cough during suction-ing, and no infusion of vasoactive agents. Measures of adequate respiratory function that are compatible with extubation have also been proposed, including a measure of rapid and shallow breathing, calculated as the respiratory rate divided by the tidal volume during spontaneous breaths. A threshold of 105 breaths/ min/L, measured after 1 minute of spontaneous breathing, provides an excellent means to predict a successful extubation, with a positive and a negative predictive value of 0.78 and 0.95, respectively.48
Most patients can be removed rapidly from mechanical ventilation once the acute illness requiring mechanical ventilation has been reversed, and over 75% of patients who meet these initial screening criteria can be extubated successfully.49 These patients can be quickly identified by a 30-minute to 2-hour trial of spontaneous breathing while they remain connected to the ventilator. Patients who appear comfortable after a trial of spontaneous breathing (respiratory rate < 35 breaths/min; heart rate < 140 beats/min; adequate arterial oxygen saturation; and no evidence of anxiety, diaphoresis, or extreme hypotension or hypertension) will likely be successfully removed from mechanical ventilation. Therefore, the concept of weaning, or slowly removing the patient from mechanical ventilation, does not apply to the majority of patients who require mechanical ventilation.
The remaining 25% of patients who require mechanical ventilation for acute respiratory failure cannot be removed rapidly from the ventilator.49 There are several options for this group of patients. The three most commonly used modes of weaning are the following:
|
Table 4 Factors to Consider in Patients Having Difficulty Being Liberated from Mechanical Ventilation |
|
Weaning parameters |
|
PaO2 a 60 mm Hg with an F^ < 50% PEEP < 5 cm H2O |
|
PaCO2 and pH in the acceptable range for patient’s lung function |
|
Spontaneous tidal volume a 5 ml/kg |
|
Vital capacity a 10 ml/kg |
|
Minute ventilation (MV) < 10 L/min |
|
Maximum voluntary ventilation double of MV |
|
Maximum inspiratory pressure (MIP) a 25 cm H2O |
|
Respiratory rate < 30 breaths/min |
|
Rapid shallow breathing index* < 105 |
|
Endotracheal tube |
|
Use largest tube possible |
|
Consider use of supplemental pressure-support ventilation |
|
Suction secretions |
|
Arterial blood gases |
|
Avoid or treat metabolic alkalosis |
|
Maintain PaO2 at 60 to 65 mm Hg to avoid blunting of respiratory drive |
|
For patients with CO2 retention, keep PaCO2 at or above the baseline level |
|
Nutrition |
|
Ensure adequate nutritional support |
|
Avoid electrolyte deficiencies |
|
Avoid excessive calories |
|
Secretions |
|
Clear regularly |
|
Avoid excessive dehydration |
|
Neuromuscular factors |
|
Avoid neuromuscular depressant drugs |
|
Avoid unnecessary corticosteroids |
|
Airway patency |
|
Use bronchodilators when appropriate |
|
Exclude foreign bodies within the airway |
|
Wakefulness |
|
Avoid oversedation |
|
Wean in morning or when patient is most awake |
*Respiratory rate divided by tidal volume during spontaneous breaths; measured after 1 min of spontaneous breathing and calculated in breaths/min/L
Fp2—fraction of inspired oxygen
PEEP—positive end-expiratory pressure
PaO2—partial pressure of oxygen in arterial blood
PaCO2—partial pressure of carbon dioxide in arterial blood
1. SIMV, which allows spontaneous breathing and diminishing numbers of mandatory breaths per minute until the patient is breathing unassisted.
2. The use of a T-piece circuit, which allows intermittent trials with total removal of mechanical support.
3. Use of decreasing levels of pressure-support ventilation.
Two large studies compared these three methods of weaning. The first one found that a gradual decrease in the level of pressure support was the most effective.50 The second study found that a daily T-piece trial was associated with the shortest duration of mechanical ventilation.49 The disparate conclusions of these two studies may in part be the result of differing criteria for extubation. Currently, it appears that both the gradual reduction in the level of pressure support and the use of intermittent T-piece trials are effective methods for weaning, but neither offers a clear advantage over the other. Gradual reduction in the number of machine-supported breaths by use of SIMV appears to be the least effective method of weaning.
Several other measures can promote successful removal from mechanical ventilation. Daily screening of the respiratory function of patients receiving mechanical ventilation, followed by trials of spontaneous breathing initiated by a respiratory therapist, can reduce the duration of mechanical ventilation.51,52 Daily interruption of sedative infusions has also been associated with decreased duration of mechanical ventilation and length of stay in the ICU.53 In addition, it has been shown that fewer diagnostic tests to assess change in mental status are performed in patients assigned to daily interruption of sedative agents. Finally, nonin-vasive ventilation has been used as a technique to expedite weaning for patients with COPD.54 Extubation and the application of noninvasive ventilation by face mask attempted after 48 hours has been associated with a significantly shorter mean duration of mechanical ventilation. For patients having difficulty being weaned from mechanical ventilation, a systematic approach to the evaluation of factors contributing to potential weaning failure is required [see Table 4].
Extubation
Usually, extubation should be performed early in the day, when full ancillary staff are available. The patient should be clearly educated about the procedure, the need to cough, and the possible need for reintubation. Elevation of the head and trunk to more than 30° to 45° improves diaphragmatic function. Equipment for reintubation should be available, and a high-humidity, oxygen-enriched gas source with a higher-than-current FjO2 setting should be available at the bedside. The patient’s airway and the oropharynx above the cuff should be suctioned. The cuff of the endotracheal tube should be deflated partially, and airflow around the outside of the tube—indicating the absence of airway obstruction—should be detected. After the cuff is deflated completely, the patient should be extubated, and high-humidity oxygen should be administered via face mask. Coughing and deep breathing should be encouraged while the examiner monitors the patient’s vital signs and upper airway for stridor. Inspiratory stridor may result from glottic and subglottic edema. If clinical status permits, treatment with nebulized 2.5% racemic epinephrine (0.5 ml in 3 ml normal saline) should be administered. If upper airway obstruction persists or worsens, reintubation should be performed. Extubation should not be reattempted for 24 to 72 hours after reintubation for upper airway obstruction. Otolaryngology consultation may be beneficial to exclude other causes of upper airway obstruction and to perform tracheostomy if upper airway obstruction persists.