Diagnosis
Clinical manifestations Patients with CTEPH usually present months or years after the initial (and often asymptomatic) embolic event(s) with symptoms of pulmonary hypertension.35 The initial event may have been diagnosed as an episode of embolism (in only 50% of cases) or may have been undiagnosed or misdiagnosed as pneumonia or another clinical entity. The reason for the delay in onset of symptoms is unknown, although it is suspected that organization of the clot and the increased pulmonary arterial pressure result in progressive remodeling of the obstructed and unobstructed pulmonary vasculature, gradually worsening the pulmonary hypertension and finally resulting in right ventricular failure.
Physical examination A pulmonary arterial flow murmur is the only finding on physical examination that is characteristic of chronic thromboembolic pulmonary hypertension. It is best heard over the lung fields while the patient holds his or her breath.
Imaging and physiologic testing The chest radiograph may be normal or may show abnormalities suggestive of pulmonary hypertension. Findings that suggest chronic thromboembolic pulmonary hypertension include asymmetrical enlargement of the pulmonary arteries, regions of hyperperfusion and hypoper-fusion, and focal fibrotic areas of old infarction that may be associated with local pleural thickening or cavitation.
The electrocardiogram may be normal or have changes of right ventricular hypertrophy or strain, right atrial enlargement, and right bundle branch block.
Pulmonary function tests will most commonly show no abnormality of lung volumes or of spirometry measurements, although about 20% of patients will have restriction, probably related to previous infarction.35 Frequently, carbon monoxide diffusion in the lung is mildly reduced; this reduction is thought not to be proportional to the degree of obstruction. Hypoxemia is common and is often worsened by exercise. The hypoxemia is the result of a combination of ventilation-perfusion inequality, low cardiac output, and, sometimes, a patent foramen ovale with a right-to-left shunt.35
The ventilation-perfusion lung scan is the best method of screening patients with pulmonary hypertension to identify those in whom the disorder may be caused by chronic throm-boembolism.35,37 Patients with chronic thromboembolism will have at least one segmental or larger perfusion defect [see Figure 5]. This pattern can also be seen with tumor or fibrosing medias-tinitis. In chronic thromboembolism, the degree of obstruction is underestimated by the defects seen on the perfusion scan.
When large perfusion defects are seen on the ventilation-per-fusion scan, additional testing is needed to confirm that the defects are caused by chronic thromboembolism. Spiral CT is a noninvasive imaging technique that can allow identification of proximal chronic clots and detect other causes of pulmonary vascular obstruction [see Figure 6].35 However, pulmonary an-giography is needed to accurately assess the full extent of pulmonary vascular obstruction and determine candidacy for pulmonary thromboendarterectomy.
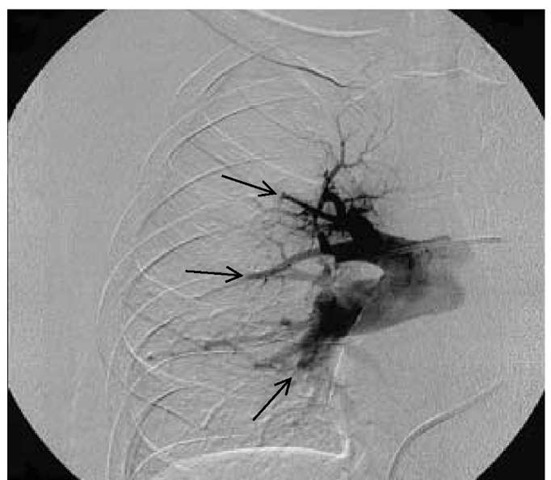
Figure 7 Pulmonary arteriogram of a patient with chronic thromboembolic pulmonary hypertension showing multiple abrupt terminations (arrows) of the pulmonary arterial branches, with no distal flow characteristic of chronic pulmonary embolism.
The use of pulmonary angiography in patients with pulmonary hypertension has been thought to be associated with a significant risk of death or adverse outcome. Use of oxygen, small amounts of nonionic contrast dye, and a limited number of injections has reduced the risk dramatically.35 Results of pulmonary angiography must be interpreted with the understanding that the patterns of pulmonary hypertension are different from those of acute embolism [see Figure 7].35 In some cases, direct visualization of the pulmonary vasculature by pulmonary angioscopy is useful to confirm the presence and extent of obstruction.35
Treatment
There is no effective medical therapy for CTEPH. Vasodilators, angioplasty of the obstructed areas, and thrombolytic therapy have been investigated in small studies and have limited utility. Preoperative anticoagulation is needed to prevent further embolism and in situ thrombosis. Placement of an inferior vena cava filter is recommended.35
Surgery for CTEPH is pulmonary endarterectomy, a procedure that is associated with significant risk and therefore should not be considered unless the patient meets criteria for surgery. The criteria used in the centers with expertise are: an elevated pulmonary vascular resistance (> 300 dynes^seocm-5), severe disability (at least NYHA class III), and surgically accessible dis-ease.35 Contraindications or factors that increase risk include significant comorbid disease, especially renal disease, inoperable coronary artery disease, other lung disease, and massive obesity. At highly experienced centers, survival has continued to improve over time: before 1990, mortality was 15.8%; from 1998 to 2002, mortality was reduced to 4.4%.38
Pulmonary thromboendarterectomy is performed through a median sternotomy, with cardiopulmonary bypass, deep hypothermia, and periods of complete arrest (because of the brisk backbleeding from the increased bronchial collateral circulation), allowing better visualization in a bloodless field.35 Dissection of the pulmonary artery starts in the proximal vessels and extends into the subsegmental branches, including the intima and some media. If an atrial septal defect or a patent foramen ovale is found, it is corrected.
Besides experiencing the usual complications associated with cardiac surgery, these patients have some specific problems, including reperfusion pulmonary edema in the previously obstructed areas, resulting in hypoxemia caused by shunting of blood from the previously unobstructed but remodeled pulmonary arterial bed into these newly opened ar-eas.35 Altered mental status is very common and seems to relate to the total circulatory arrest time.
Patients who are not candidates for pulmonary thromboen-darterectomy may be considered for medical therapy with drugs used to treat other forms of pulmonary hypertension, although experience to date is limited.39-41
Prognosis
Without surgery, the survival rate for patients with CTEPH is poor. In patients whose mean pulmonary arterial pressure is 40 to 50 mm Hg, 5-year survival is 30%; in those whose pulmonary arterial pressure is greater than 50 mm Hg, 5-year survival is 10%.35
Mortality associated with pulmonary thromboendarterecto-my ranges from 5% to 24% and is lowest in the institutions with the greatest experience. The patients who survive have improvement of functional status from NYHA class III or IV to NYHA class I or II.35 The right heart recovers quickly, and the pulmonary arterial pressure may continue to fall for up to 1 year after surgery. Many patients are able to return to work.
Conditions associated with pulmonary arterial hypertension
Collagen Vascular Diseases
Pulmonary hypertension with little or no parenchymal lung disease is a life-threatening manifestation of the collagen vascular diseases.42 The clinical course and pathologic changes are similar to those of IPAH. The pathophysiology is unknown, although autoantibodies, immunoglobulin, and complement have been found in the vessel walls, suggesting involvement of immune complexes.43 Patients with scleroderma, particularly those with CREST syndrome (calcinosis, Raynaud phenomenon, esophageal involvement, sclerodactyly, and telangiectasias) have a high incidence of pulmonary hypertension; the incidence ranges from 2% to 35% in patients with scleroderma to 50% in those with CREST syndrome. The incidence in patients with other disorders varies from 23% to 53% in those with mixed connective tissue disease to 0.5% to 14% in those with systemic lupus erythematosus; it is rare in those with rheumatoid arthritis, Sjogren syndrome, and dermatomyositis. Treatment of pulmonary hypertension in patients with collagen vascular diseases should be patterned after the protocol for IPAH detailed above.25 44 45
The role of immunosuppressive therapy in patients with pulmonary hypertension secondary to collagen vascular diseases is unclear. Sanchez and coworkers42 reviewed the literature and found improvement in pulmonary hemodynamics with various immunosuppressive regimens in seven of 11 published cases. There have been no randomized, controlled trials with these agents, and this approach is not recommended as the primary treatment strategy.
Although patients with pulmonary hypertension secondary to collagen vascular disorders are sometimes excluded from consideration of lung transplantation, transplants have been performed in such patients, with prolonged survival.46
Several disorders are associated with vasculitis involving the pulmonary vasculature; however, pulmonary hypertension associated with vasculitis is rare. The exceptions to this rule are Takaya-su disease [see 15:VIII Systemic Vasculitis Syndromes], in which pulmonary arteritis and hypertension are common findings,47 and rheumatoid arthritis48 and systemic lupus erythematosus, in which pulmonary hypertension occurs much less commonly.49
Pulmonary Veno-occlusive Disease
Pulmonary veno-occlusive disease is a rare but distinct form of pulmonary hypertension characterized by obstruction of the small intrapulmonary veins.50 About one third of patients are children, and there are some cases that seem to be related to HIV infection, use of chemotherapy drugs, or bone marrow transplantation.
Patients with this disorder experience increasing dyspnea, sometimes with hemoptysis. Findings on chest radiography suggest left ventricular failure; such findings include enlarged pulmonary arteries, Kerley B lines, pulmonary edema, and pleural effusions. The diagnosis should be considered when these radi-ographic findings are associated with no echocardiographic evidence of left ventricular dysfunction, mitral valvular disease, or obstruction to flow in the left atrium. CT scan of the chest can provide support for the diagnosis51 and can help exclude obstruction of the pulmonary veins in the mediastinum (fibrosing mediastinitis or tumor). The diagnosis can be confirmed by open lung biopsy, which will show the combination of chronic congestion, pulmonary hypertensive changes in the pulmonary arteries, and narrowing or occlusion of the pulmonary veins by eccentric or concentric intimal fibrosis.
Therapies that are used in IPAH are inconsistently successful in veno-occlusive disease, and epoprostenol in particular has been associated with the development of pulmonary edema. Accordingly, lung transplantation should be considered once the diagnosis is established.50,52
Hematologic Disorders
Pulmonary hypertension is common in adults with sickle cell disease and most likely is a complication of hemolysis resulting from interference with the nitric oxide regulatory pathway [see 5:IV Hemoglobinopathies and Hemolytic Anemias].
Patients with chronic myeloproliferative disorders,54 amyloi-dosis,55 and the POEMS (polyneuropathy, organomegaly, endocrin-opathy, monoclonal gammopathy, and skin changes) syndrome56 have been noted to have complicating pulmonary hypertension.
Pulmonary Arteriovenous Malformations
Abnormal, direct communications between branches of the pulmonary arteries and veins, producing a right-to-left shunt, are called pulmonary arteriovenous malformations (AVMs). Such lesions may be acquired (e.g., as a result of trauma or as a complication of cirrhosis) [see 4:IX Cirrhosis of the Liver] or congenital.57
Congenital pulmonary AVMs can be either single or multiple. They may be isolated to the lung or, much more commonly, can occur as part of an autosomal dominant syndrome of widespread AVMs in skin, mucous membranes, lungs, and other internal organs. The autosomal dominant syndrome is known as Osler-Weber-Rendu disease or hereditary hemorrhagic telan-giectasia (HHT)57 [see 5:X1II Hemorrhagic Disorders].
Diagnosis Pulmonary AVMs may produce severe symptoms or complications, but most patients are asymptomatic. The diagnosis is suggested by solitary or multiple pulmonary nodules found on routine chest radiography.58 Dyspnea is the most common symptom, occurring at first with exercise and later at rest. Dyspnea that worsens in the upright position and improves on reclining (platypnea) has been seen in these patients. It may be caused by worsening of the right-to-left shunt in the lung bases with the patient in the upright position, producing hypox-emia (orthodeoxia). Hemoptysis, usually mild and related to bronchial mucosal telangiectasia, is the second most common symptom. Neurologic complaints are also seen.58,59 Headache, tinnitus, seizures, symptoms that mimic transient ischemic attack, and completed stroke may relate to complicating poly-cythemia, paradoxical embolus, or brain abscess. Patients with HHT may have bleeding from AVMs outside the lungs, which is often sufficient to produce anemia. Physical findings include mucous membrane telangiectasia in the patients with HHT, cyanosis, digital clubbing, and intrapulmonary bruits that increase with inspiration. Patients with pulmonary AVMs have hypoxemia, with the degree of hypoxemia being proportional to the magnitude of the shunt. As a consequence, chronic respiratory alkalosis and polycythemia may occur. Pulmonary function tests are normal except for a decreased diffusing capacity.
The diagnosis is most often suggested by the finding on chest radiograph of a well-defined solitary pulmonary nodule or multiple nodules with a feeding artery, draining vein, or both. Echo-cardiography with contrast can also suggest the diagnosis.58 Spiral CT with contrast will confirm the vascular nature of lesions and may detect others that were not obvious from standard radiography. The definitive diagnostic procedure, however, is pulmonary angiography, which allows demonstration of all significant lesions; this is important in planning therapy.59,60
Treatment The natural history of pulmonary AVMs is poorly understood. In asymptomatic patients, the treatment decisions must be made with the recognition of the risk of hemoptysis, paradoxical embolization, and brain abscess.
The treatment options include thoracotomy with selective resection or percutaneous embolization. In patients with solitary or unilateral lesions, resection can be performed with minimal morbidity and mortality.61 The only consideration is the possibility of the development or persistence of pulmonary hypertension after the low-resistance AVMs are removed in patients whose pulmonary vasculature has become remodeled in response to the high flow through the AVMs, or who have the ALK-1 genetic mutation associated with HHT and pulmonary hypertension.62 In such patients, percutaneous balloon obstruction of the AVMs allows preoperative measurement of pulmonary hemodynamics.
The techniques and safety of percutaneous embolization have been improved. With the use of detachable balloons and metal coils, single or multiple AVMs can be treated.61 The main risk is systemic embolization of the obstructing material. This technique is rapidly replacing surgery as the treatment of choice.
Pulmonary Arterial Aneurysms
Aneurysms of the pulmonary arteries are very rare.63 They are seen as congenital anomalies, often along with abnormalities of the other great vessels and heart; as part of connective tissue disorders (e.g., Marfan syndrome); as a consequence of trauma (pseudoaneurysms caused by Swan-Ganz catheters may be confused for pulmonary aneurysms), infection (syphilis, tuberculo-sis, pyogenic bacteria, or fungi), or immunologic disorders (Beh-get disease64 or Hughes-Stovin syndrome); and in association with pulmonary diseases such as pulmonary hypertension or bronchiectasis.
In many cases, pulmonary arterial aneurysms are asymptomatic. However, cough, dyspnea, and particularly hemoptysis can be seen. Rupture of the aneurysm into an airway can be associated with sudden, massive, and usually fatal hemoptysis. Dissection of the pulmonary artery can also occur. The diagnosis can be made from a contrast-enhanced CT scan or by pulmonary angiography.
Aneurysms of the main and proximal right and left pulmonary arteries can be surgically repaired.65 Aneurysms in smaller pulmonary arteries can be removed by surgical resection of the involved area of lung.
Other causes of pulmonary hypertension
Residence at high altitude may be associated with the development of pulmonary hypertension.66 This response is maladap-tive and occurs in only a small portion of the population living at high altitude.67 In these patients, moving to a lower altitude best reduces pulmonary arterial pressure. Patients with obstructive sleep apnea without other forms of lung disease often have complicating pulmonary hypertension.68 Development of pulmonary hypertension is associated with greater body mass index and degree of daytime hypoxemia, small airway closure during tidal breathing, and heightened pulmonary pressor responses to hypoxia and increased pulmonary blood flow.69,70 Treatment of sleep apnea is associated with improvement in pulmonary hemodynamics70 [see 14:VI Ventilatory Control during Wake-fulness and Sleep]. Because these patients are often sedentary, obese, and have risk factors for left-sided heart disease, the clinical evaluation should assess for recurrent pulmonary thrombo-embolic disease and left ventricular dysfunction (systolic and/or diastolic) as underlying causes for the pulmonary hypertension.
Compression or stenosis of the pulmonary arteries or veins in the mediastinum by tumor or fibrosing mediastinitis [see 14:IX Disorders of the Pleura, Hila, and Mediastinum] or stenosis of multiple peripheral segments of the pulmonary artery can produce precapillary or postcapillary pulmonary hypertension. These disorders can usually be diagnosed by spiral CT or pulmonary angiography.
A rare cause of pulmonary hypertension is pulmonary capillary hemangiomatosis, a disorder that clinically mimics or can be a component of pulmonary veno-occlusive disease.1,71 The lung biopsy specimens of these patients have patchy regions of severe congestion that contain capillary-sized blood vessels that appear to invade the walls of the pulmonary veins and, to a lesser degree, the pulmonary arteries. A CT scan can assist in differentiating this disorder from IPAH.72 The only effective treatment is lung transplantation.