Cor Pulmonale
Definition
Cor pulmonale is the term used for pulmonary hypertension resulting from disorders of the pulmonary parenchyma, the thoracic cage, or the neuromuscular system, excluding congenital heart disease and disorders of the left side of the heart. Cor pul-monale can occur acutely in settings of rapid-onset right ventricular overload, or chronically with the slow onset of pulmonary hypertension.
Acute cor pulmonale
Pathogenesis
The right ventricle normally pumps against a low afterload, even when cardiac output is dramatically increased by exercise. In response to an acute increase in pulmonary vascular resistance, the right ventricle distends, producing an increase in right ventricular systolic and diastolic volume, but is unable to generate high pressures (the maximal pressure generated is usually approximately 40 mm Hg). If the right ventricle cannot adequately compensate, increases in right ventricular end-diastolic pressure (RVEDP) and right atrial pressure occur, producing acute right heart failure. Additionally, the reduced cardiac output from the right ventricle to the left ventricle and the shift of the interventricular septum toward the left ventricle cause a re- duction in left ventricular filling, resulting in systemic hypoper-fusion. Decreased coronary perfusion caused by low systemic diastolic pressure further reduces the ability of the right ventricle to overcome the added resistance, producing a rapid decline to death.
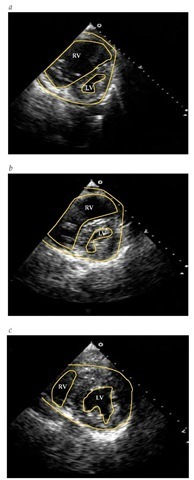
Figure 4 (a) Echocardiogram of a patient with chronic pulmonary hypertension. (b) Echocardiogram of a patient with acute pulmonary hypertension. (c) Echocardiogram of the patient shown in Figure B after clot lysis. (LV—left ventricle; RV—right ventricle)
The disorders that cause acute cor pulmonale are diseases that produce sudden obstruction of the pulmonary vasculature, such as massive pulmonary thromboembolism6; acute embolism caused by other materials, such as air, bone marrow, fat, amniot-ic fluid, or tumor; or obstruction of the microvasculature caused by high airway pressure or destruction, as is seen in acute respiratory distress syndrome.7
Diagnosis
In the setting of acute respiratory failure or shock with evidence of acute right heart failure, immediate bedside echocar-diography can demonstrate acute cor pulmonale. Features that are seen include pulmonary hypertension (usually mild), right ventricular dilatation without hypertrophy, tricuspid regurgita-tion, septal flattening or paradoxical septal motion, and left ventricular diastolic dysfunction [see Figure 5].8
Treatment
Rapid recognition of acute cor pulmonale and relief of the pulmonary vascular obstruction, if possible, are key to survival for patients with acute cor pulmonale. For example, thrombolyt-ic therapy for patients with acute massive pulmonary embolism may result in complete resolution of the acute cor pulmonale [see Figure 6].9
Chronic cor pulmonale
Pathogenesis
In response to a chronic increase in pulmonary vascular resistance, the right ventricle will distend and undergo hypertrophy. When the ability of the right ventricle to compensate is overwhelmed, increases in RVEDP and right atrial pressure occur, resulting in right heart failure.
The pulmonary hypertension that characterizes chronic cor pulmonale is produced by increased pulmonary vascular resistance caused by varying combinations of pulmonary vascular destruction or obstruction, dynamic vasoconstriction caused by hypoxia or acidosis, and remodeling of the pulmonary vascula-ture. Some of these changes (e.g., hypoxic vasoconstriction and some remodeling) are reversible with therapy, whereas others (vascular bed destruction) are not.
Diagnosis
Early detection of chronic cor pulmonale may be difficult because the manifestations of the underlying lung disease dominate the clinical picture. The clinical features and evaluation are the same as those described for pulmonary hypertension [see Pulmonary Hypertension, above] Echocardiography has become the most useful means of detecting right heart changes caused by pulmonary hypertension.
Treatment
The nature and severity of the underlying lung disease determine the outcome of patients with chronic cor pulmonale. For example, cor pulmonale secondary to sleep apnea may be entirely reversible with treatment directed at the underlying ventilato-ry disorder, whereas cor pulmonale caused by idiopathic pul-monary fibrosis is often irreversible. In patients with COPD, an increased incidence of right ventricular involvement may correlate with increasing severity of lung dysfunction.
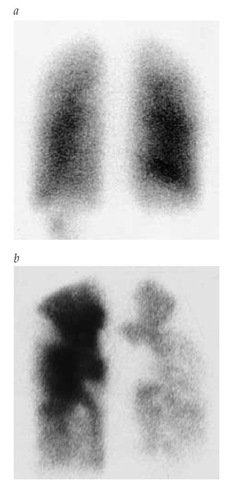
Figure 5 Perfusion lung scans of a patient with primary pulmonary hypertension showing homogeneous perfusion (a) and of a patient with chronic thromboembolic pulmonary hypertension, showing large bilateral perfusion defects (b)
The most important treatment of chronic cor pulmonale is the treatment of the underlying lung disease [see 14:III Chronic Obstructive Diseases of the Lung, 14:V Chronic Diffuse Infiltrative Lung Disease, 14:VI Ventilatory Control during Wakefulness and Sleep, and 14:VII Disorders of the Chest Wall]. In patients with hypoxemia, controlled-flow supplemental O2 should be given at rest, during exercise, and during sleep to maintain O2 saturation above 90%. Patients should be monitored for acute increases in arterial carbon dioxide pressure (PaCO2).
Diuretics are useful in reducing the edema, ascites, and liver congestion associated with cor pulmonale, but these agents must be used carefully to avoid reducing right ventricular filling pressures, which may lead to decreasing cardiac output. Phlebotomy in patients with secondary polycythemia10 and noninvasive mechanical ventilation of patients with chronic respiratory failure11 may also improve hemodynamics.
Although vasodilator agents such as calcium channel blockers block hypoxic pulmonary vasoconstriction and cause pulmonary vasodilatation, the impairment in gas exchange in patients with chronic cor pulmonale negates any potential positive effect of these drugs. Epoprostenol (prostacyclin) and three of its analogues have been studied in several types of chronic pulmonary hypertension and are approved for use in PAH, but they have not been studied extensively in cor pulmonale.12 Except in cases of coexistent left ventricular failure, clinical studies do not support the use of digitalis in patients with cor pulmonale; in addition, use of digitalis may be associated with greater toxicity.
Intrinsic Pulmonary Vascular Diseases
Idiopathic pulmonary arterial hypertension
Idiopathic pulmonary arterial hypertension (IPAH), formerly known as primary pulmonary hypertension (PPH), is a condition in which the pulmonary vasculature is the exclusive site of the pulmonary hypertension.1,13 By definition, other causes of pulmonary hypertension are excluded.
Epidemiology
IPAH is rare, having an estimated incidence of 1 to 2 per million people a year. Although the disease can occur at any age, the mean age at diagnosis is 36 years.13 The disease is more frequent in females than in males (ratio, 1.7:1 to 3.5:1) and is equally represented in all races. Approximately 6% to 12% of cases are familial, now termed familial pulmonary artery hypertension (FPAH).1,14
In the United States, the mortality from IPAH rose substantially from 1979 to 1996, possibly because of the introduction of anorexigens (see below).
Etiology and Pathogenesis
The cause of IPAH is unknown, although there are several clinical conditions associated with its development. For example, the ingestion of certain drugs or other materials has been associated with IPAH.16 In the 1960s and again in the 1990s, ingestion of appetite suppressants such as aminorex, fenfluramine, and dexfenfluramine were associated with an increased incidence of pulmonary hypertension.17 Additionally, ingestion of contaminated rapeseed oil, L-tryptophan, amphetamines, and cocaine are associated with IPAH.16 Other conditions associated with IPAH are splenectomy/asplenia, portal hypertension, and HIV infection.
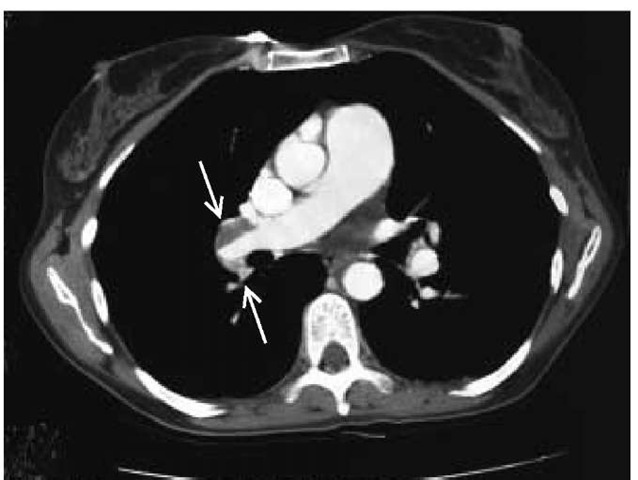
Figure 6 Spiral CT scan of the chest of a patient with chronic thromboembolic pulmonary hypertension showing large defects in the right main pulmonary artery (arrows).
As is true of many diseases, it is thought that PAH in general, and IPAH in particular, results from an inciting factor in a genetically susceptible individual. Studies of inheritance have suggested an autosomal dominant pattern with markedly reduced penetrance in FPAH. The gene for familial pulmonary hypertension, PPH1, has now been identified as heterogeneous germline mutations of the gene coding for the bone morphogenic protein receptor 2 (BMPR2).20 The exact mechanisms accounting for how abnormalities of this receptor produce pulmonary hypertension are being investigated.
Studies of the pulmonary vasculature in IPAH suggest en-dothelial injury and dysfunction occur early in the process.22 In IPAH, expression of endothelial nitric oxide synthetase is reduced (nitric oxide is a pulmonary vasodilator) and expression of endothelin 1, a potent pulmonary vasoconstrictor and a mito-gen, is increased. There is an excess of thromboxane (a vasoconstrictor and potent stimulus for platelet aggregation) relative to prostacyclin, a pulmonary vasodilator. Other vasoactive mediators, such as serotonin, another pulmonary vasoconstrictor, may also play a role, especially in pulmonary hypertension associated with appetite suppressants, which inhibit serotonin reuptake and increase expression of its transporter. Abnormal a-padreno-ceptor affinity and responsiveness may produce downstream signaling events that cause defects in ion channel activity and control of intracellular calcium and could contribute to vasocon-striction and to smooth muscle proliferation and growth.23 As a consequence of the endothelial dysfunction and resultant pulmonary vasoconstriction, intimal proliferation, smooth muscle hyperplasia and hypertrophy, and other remodeling phenomena occur, further increasing pulmonary vascular resistance.19 In situ thrombosis may also play a role in endothelial injury and vascular obstruction.
Diagnosis
The diagnosis of IPAH can be made when clinical findings (e.g., raised pulmonary artery pressure, dyspnea, evidence of right heart failure) [see Pulmonary Hypertension, above, and Figure 3] are present and other causes of pulmonary hypertension have been excluded.
Treatment
Medical therapy Current treatment options slow the progression of the disease but do not halt it. Patients with IPAH should receive long-term warfarin anticoagulation therapy to achieve an INR (international normalized ratio) of 1.5 to 2.5, and they should avoid medications that interfere with warfarin me-tabolism.13 Medications that worsen pulmonary hypertension or right heart function (e.g., decongestants, beta blockers) should also be avoided. Symptom-limited physical activity should be encouraged. Pregnancy is poorly tolerated and should be prevented (oral contraceptives should not be used, because they may increase the risk of thrombosis). Invasive medical procedures should be avoided whenever possible. Oxygen should be used for resting or exercise-induced hypoxemia or if the patient will be exposed to high altitudes, such as on an airplane flight.
Diuretics are useful to help control edema and ascites. Use of digoxin is controversial.24 Vasodilators may be helpful for only a select portion of patients with pulmonary hypertension who have evidence of significant vasoreactivity, generally considered to be 10% of those with IPAH and very few with other forms of PAH. For patients who have an acute vasodilator response to the agents used (i.e., inhaled nitric oxide, inhaled iloprost, intravenous epoprostenol, or intravenous adenosine), a trial of a calcium channel blocker, such as nifedipine or diltiazem, should be initiated. The patient should be monitored closely during this time because some patients will deteriorate despite this therapy. If the patient responds, the therapy can be continued and titrated to maximum benefit. Patients should be warned that abrupt withdrawal of therapy can lead to rebound pulmonary hypertension that can be fatal.24
Patients who do not show a vasodilator response during right heart catheterization should be started on one of the other available agents, including the oral endothelin receptor antagonists (e.g., bosentan), subcutaneous or intravenous treprostinil, inhaled iloprost, or intravenous epoprostenol.13,25-27 Epoprostenol must be given intravenously and, because of its 3- to 5-minute half-life, delivered by continuous infusion. Abrupt discontinuance of therapy may be required because of drug-delivery system malfunction or I.V. access infections. Minor side effects include headache, jaw pain, rash, diarrhea, and joint pain. The dose must be gradually increased over time to maintain maximum benefit. Epoprostenol appears to have effects that go beyond vasodilation and may include the decreased production of endogenous vasoconstrictor substances and antiplatelet and an-tiproliferative properties that seem to ameliorate what previously appeared to be irreversible vascular changes.28 Bosentan is administered orally twice daily. The major toxicity is hepatic, which necessitates monthly monitoring of liver function. Tre-prostinil administered subcutaneously produces site pain that often limits its usefulness. Inhaled iloprost obviates the need for intravenous delivery of prostanoid medication; however, it must be administered by nebulization six to nine times daily.
Surgical therapy Creation of a small interatrial communication by percutaneous balloon atrial septostomy may result in decompression of the right ventricle and improvement in symptoms related to right heart failure.29 Indications include recurrent syncope and right heart failure despite maximum medical therapy, deterioration despite maximum medical therapy while the patient is awaiting transplantation, and exhaustion of all other options. Because right-to-left shunting at the atrial level occurs, hypoxemia worsens but is usually well tolerated in patients without severe preprocedure hypoxemia.
Lung transplantation Lung transplantation is indicated for patients in whom IPAH has progressed despite optimal medical and surgical therapy. In most centers, heart-lung transplantation is no longer performed because it was found that the right ventricle recovered both form and function when pressure fell after even single-lung transplantation. Indications for referral for transplant evaluation include New York Heart Association (NYHA) functional class III or IV despite medical therapy29; failure of epoprostenol therapy and the occurrence of severe side effects from epoprostenol are additional indications for referral for transplant evaluation. These guidelines take into consideration the course of the disease and the waiting time for transplantation.
The surgical mortality for patients with IPAH is higher than that for patients receiving lung transplantation for other forms of pulmonary disease,30 partly because of the greater complexity of the transplant surgery. The 1-year survival for patients receiving lung transplantation for IPAH is 65%; the 3-year survival is 55%; and the 5-year survival is 44%. No randomized study of medical therapy versus transplantation has been performed. Comparison of survival data from different studies suggests that survival may be higher with medical therapy than with transplantation, although it is likely that only the more severely ill patients received lung transplantation.31 Recurrences of IPAH after lung transplantation has not been reported.
Prognosis
A National Institutes of Health (NIH) registry of patients with IPAH in the 1980s defined the natural history of the disease.32 The mean life expectancy from diagnosis was 2.8 years, and the 5-year survival was 22% to 38%. Patients younger than 14 years and older than 65 years had lower survival, as did patients with more severe symptoms. Patients with acute responses to vasodilators had a better prognosis. This study also developed a formula, utilizing data from right heart catheterization (right atrial pressure, cardiac index, and mean pulmonary arterial pressure), that predicted survival of patients before the current era of efficacious medical therapy and lung transplantation. With these new therapies, the natural history of the disease has changed. A recent evaluation study identified African-American and Asian descent as factors associated with an increased risk of death in patients with IPAH; cardiac function and acute reactivity of the pulmonary vascular bed remained strong independent predictors of outcome.33
Chronic thromboembolic pulmonary hypertension
Epidemiology
The epidemiology of chronic thromboembolic pulmonary hypertension (CTEPH) is unknown. An estimated 0.1% to 4% of patients with acute pulmonary embolism will experience chronic pulmonary hypertension, suggesting that several thousand cases occur each year in the United States.34 There are conflicting data on the male-to-female ratio. The disease has been seen in adults of all ages, but more than 50% of patients are younger than 45 years. The reason for the development and lack of resolution of pulmonary emboli in these patients is unknown. A minority of the patients is found to have hypercoagulability states, such as deficiencies of protein C or S or of antithrombin III. About 10% will have circulating lupus anticoagulant.35 The incidence of factor V Leiden and other hypercoagulable syndromes has not been adequately studied. Abnormalities of the fibrinolyt-ic system have been sought, but no consistent patterns have been detected.35 Embolization of so-called aged clot, lack of therapy for the initial episode of embolism, and recurrent emboli have all been hypothesized. Medical conditions that may be associated with an increased risk for the development of CTEPH are splenectomy, ventriculo-atrial shunt for the treatment of hydro-cephalus, and chronic inflammatory disorders such as osteomyelitis and inflammatory bowel disease.36
Pathogenesis
CTEPH results from the organization (rather than lysis) of the clots from a single massive episode of pulmonary embolism or multiple episodes of pulmonary embolism. As a consequence of obstruction and distortion of the proximal pulmonary vascula-ture, the pulmonary vascular resistance is increased, and over time, pulmonary hypertension develops and right ventricular function worsens.