Photochemotherapy
Photochemotherapy with PUVA is indicated for patients with extensive, disabling psoriasis that has failed to respond to conventional forms of therapy, including conventional or narrowband UVB phototherapy. PUVA therapy entails the administration of the photosensitizing drug methoxsalen (8-methoxypso-ralen)—in an oral dose or by soaking in a tub containing methoxsalen or applying topical methoxsalen—followed by exposure of the patient to high-intensity longwave ultraviolet light in a walk-in irradiation chamber. The initial UVA dose (in joules/cm2) is based on the patient’s skin type and calculated in accordance with established protocols.60
Although its therapeutic effect is local, PUVA is a systemic treatment in which photoactivated methoxsalen binds to epidermal DNA, forming monofunctional and bifunctional adducts. It has been postulated that the resulting interference with epidermal mitosis is one of the mechanisms of action of PUVA therapy for psoriasis, although effects on immune function in the skin play an important role.
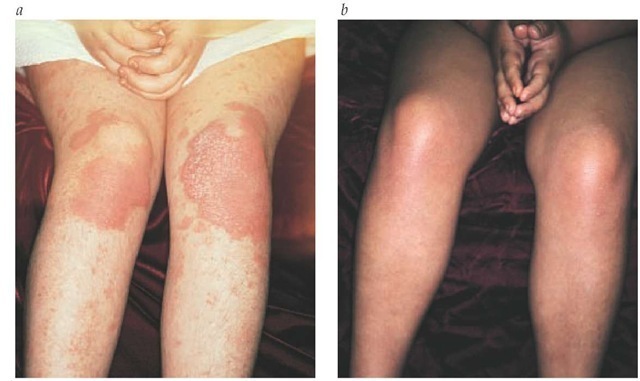
Figure 10 Psoriasis in a child before (a) and after (b) phototherapy.
The efficacy of oral PUVA therapy has been established by several multicenter clinical trials.61 A course of PUVA therapy administered two or three times weekly resulted in significant clearing of psoriasis lesions in approximately 90% of patients within a mean of 25 total treatments. After the initial course, a tapering maintenance regimen is instituted, and PUVA therapy is eventually discontinued. In most patients, psoriasis recurs months to years after PUVA is discontinued, indicating that this therapy is palliative rather than curative.
Side Effects
Acute side effects caused by phototoxicity, such as erythema and blistering, are dose related and can therefore be controlled. Pruritus, usually associated with dryness of the skin, is fairly common and can be alleviated by the use of emollients and oral antihistamines. Nausea may follow ingestion of methoxsalen. Of greater concern are the potential long-term side effects, particularly carcinogenicity. Although the FDA has approved the use of PUVA to treat psoriasis, patients must be closely monitored for long-term side effects. A multicenter study of more than 1,300 PUVA-treated patients in the United States who were evaluated after 1 to 3 years of follow-up revealed a significant increase in the number of squamous cell carcinomas (SCCs) in those patients with a history of exposure to ionizing radiation or a history of skin cancer.62 A higher-than-expected ratio of SCCs to basal cell epitheliomas and an excess of SCCs in areas of the body that were not exposed to the sun were significant findings of the study. A 5.7-year follow-up study of the original cohort group revealed a dose-dependent increase in the risk of SCC.63 There was only a slight increase in the risk of basal cell carcinoma in these patients. The risk of SCC was almost 13-fold higher in patients who had received high cumulative doses of PUVA than in patients who received low-dose therapy.
A follow-up study of the surviving members of that cohort, at least 15 years after original treatment, again assessed the risk of skin cancers. Of great concern was a small but statistically significant increase in the incidence of malignant melanoma.64 Because that increase did not become apparent until after a period of at least 15 years, there is great concern that high rates of melanoma will occur in patients who began PUVA therapy years ago. Fortunately, this has not happened thus far.
Studies in animals suggest that PUVA may have ocular side effects. Methoxsalen has been detected in the lenses of rats after they have ingested the drug; subsequent exposure to UVA enhances such ultraviolet-induced changes as cataracts.65 The risk of ocular toxicity and possible retinal damage is of particular concern in young persons, whose lenses transmit more UVA than the more opaque lenses of older persons, and in aphakic persons, in whom lenses are absent.66 The use of UVA-opaque goggles during PUVA treatment sessions is extremely important. Glasses that block UVA must be worn from the time that methoxsalen is administered throughout the rest of the day. Some investigators advise protection of the eyes the day after therapy. Thus far, studies of patients treated with PUVA have not revealed an increase in the incidence of cataracts.
Systemic therapy
Methotrexate
Short-term use of the antimetabolite methotrexate can be an extremely effective treatment for psoriasis. Methotrexate is indicated for patients who do not respond adequately to phototherapy and for patients with psoriatic arthritis.
The source of methotrexate’s efficacy against psoriasis was once thought to be its antimitotic effect on proliferating ker-atinocytes. However, tissue culture studies have suggested that activated lymphoid cells in the lymph nodes, blood, and skin are a likely target of methotrexate; proliferating macrophages and T cells are 100 times more sensitive to methotrexate than are proliferating epithelial cells.67 These findings may be relevant to the mechanism of action of methotrexate in other immunologically based disorders, including psoriatic arthritis, rheumatoid arthritis, and Crohn disease.
Dosage Methotrexate is best given in a single weekly oral dose of up to 30 mg or in three divided doses at 12-hour intervals during a 24-hour period (e.g., at 8:00 A.M., at 8:00 P.M., and again at 8:00 A.M.).
Hepatoxicity and liver biopsy The use of liver biopsy has been advocated for monitoring patients with psoriasis who are receiving methotrexate. This recommendation is controversial, however; critics point out that liver biopsies are not routinely performed in patients with rheumatoid arthritis who are undergoing treatment with methotrexate.68 Nevertheless, a review of the literature clearly shows that patients with psoriasis who are treated with methotrexate are more likely to develop hepatic fi-brosis, possibly because of their underlying disease or because of the concomitant treatments they are given.
Current guidelines call for the use of liver biopsy in patients with psoriasis who have received a cumulative dose of 1 to 1.5 g of methotrexate and who do not have a history of liver disease or alcoholism. Biopsy should be performed early in the course of treatment in patients with a history of hepatitis C, alcoholism, or other liver disease. Other risk factors for hepato-toxicity are obesity, diabetes, and abnormalities on liver function testing.
Pathologic liver changes caused by methotrexate therapy have been graded as follows: grade I, normal liver histology or mild fatty infiltration; grade II, moderate to severe fatty infiltration with portal tract inflammation and necrosis; grade IIIA, mild fibrosis; grade IIIB, moderate to severe fibrosis; and grade IV, cirrhosis. Methotrexate should be discontinued in patients with grade IIIB or IV pathologic liver changes. The importance of strict adherence to current guidelines for the administration of methotrexate is emphasized by the occurrence of methotrexate-induced cirrhosis necessitating liver transplantation in three patients with long-term psoriasis who did not undergo serial liver biopsies.69
Other side effects In addition to hepatotoxicity, other side effects of methotrexate therapy include bone marrow suppression, nausea, diarrhea, and stomatitis. Methotrexate is terato-genic and can cause reversible oligospermia. Pneumonitis can occur early in the course of treatment if methotrexate is administered in oncologic doses. Evaluation by tests of liver function, renal function, and blood elements must be made before and throughout the course of methotrexate therapy.
Certain drugs increase the toxicity of methotrexate by reducing renal tubular secretion; these drugs include salicylates, sul-fonamides, probenecid, and penicillins. Other drugs increase toxicity by displacing methotrexate from its binding sites on plasma proteins; these drugs include salicylates, probenecid, barbiturates, and phenytoin. Many of the NSAIDs and trimetho-prim-sulfamethoxazole enhance methotrexate toxicity.68 Cases of pancytopenia after low-dose methotrexate therapy underscore the hazards of using this drug in patients with renal insufficiency or in patients who are concomitantly receiving drugs that increase methotrexate toxicity.70
Contraindications to treatment with methotrexate and indications for stopping treatment should be heeded. Constant medical supervision is necessary, and therapy must be stopped at once if toxicity develops.
Acitretin
Indications and dosage Acitretin, an oral retinoid, has FDA approval for the treatment of plaque psoriasis. It is highly effective in the treatment of pustular psoriasis and can be very effective as monotherapy for erythrodermic psoriasis. For plaque-type and guttate psoriasis, however, acitretin is most useful in combination with other treatments, particularly UVB and PUVA phototherapy.71,72 Acitretin is initiated 1 to 2 weeks before UVB or PUVA therapy is started. With combination treatment, symptoms resolve much more quickly. Doses of only 10 to 25 mg daily are effective, thus minimizing retinoid side effects.71,72 When used as monotherapy, acitretin is prescribed in doses of 25 mg daily, which can be increased to 50 mg a day or higher.
Side effects Acitretin side effects are dose related and are common with doses above 25 mg daily. Hair loss, cheilitis, desquamation of the palms and soles, sun sensitivity, and peri-ungual pyogenic granulomas are among the mucocutaneous side effects. Hyperlipidemia is common but is easily controlled with lipid-lowering agents. Elevations in liver enzyme levels can occur, and enzyme levels must be monitored. Serial liver biopsies have not demonstrated hepatic fibrosis in patients treated with oral retinoids.73
Acitretin poses a significant risk of teratogenicity. Characteristic retinoid birth defects occur in a high proportion of fetuses exposed to even small amounts of the drug in utero. Acitretin is eliminated from the body much more quickly than its prodrug etretinate. In the presence of alcohol, however, acitretin is converted back to etretinate,74 raising concerns that women of child-bearing age who take acitretin and who later become pregnant would then be at risk for exposing their fetus to acitretin’s terato-genic effects. The FDA therefore requires that acitretin not be given to women planning a pregnancy within 3 years.
Long-term side effects of oral retinoids include calcification of ligaments and tendons and osteoporosis.75,76 The long-term safety of etretinate, acitretin’s prodrug, was examined in a 5-year prospective study of 956 patients with psoriasis. The investigators concluded that with appropriate patient selection and monitoring, there was no substantially increased risk of side effects related to cardiovascular disease, cancer, diabetes, cataracts, and inflammatory bowel disease. Although joint symptoms improved in some patients, more patients had joint problems associated with etretinate. Etretinate also caused short-term changes in liver enzyme levels in some patients and, in rare cases, caused acute hepatitis. The long-term risk of liver disease and cirrhosis with etretinate, however, was less than that associated with comparable periods of methotrexate.77
Cyclosporine
Cyclosporine in a microemulsion formulation was approved by the FDA for the treatment of psoriasis after extensive worldwide experience. In dosages of 2.5 to 5 mg/kg/day, cyclosporine is highly effective for psoriasis. Even at such doses, however, it may be associated with significant side effects, which have limited its use in patients with severe or refractory disease.
Indications and dosage Cyclosporine is indicated for patients in whom phototherapy or methotrexate therapy has failed. The microemulsion formulation of cyclosporine is better absorbed than earlier formulations. It is available in gel capsules of 25 and 100 mg and is most commonly taken in divided doses twice daily. At dosages of 5 mg/kg/day, a response is usually seen within 4 weeks, and some patients respond as quickly as 1 week. It should be noted that in the United States, the package insert for cyclosporine recommends an upper dosage limit of 4 mg/kg/day, although worldwide experience regarding the efficacy and safety of this drug has established an upper limit of 5 mg/kg/day.78 In the United States, the maximum FDA-approved duration of treatment of cyclosporine is 1 year.
Side effects Cyclosporine is associated with a number of side effects that are easily managed; other side effects are of greater concern. Hypertrichosis, tremors, paresthesias, headache, gingival hyperplasia, joint pain, and fatigue can occur. Elevations in serum lipid levels and minor elevations in liver enzyme levels are also common. Hypomagnesemia may require magnesium supplementation. The most serious common side effects are hypertension and nephrotoxicity. Hypertension can be managed by lowering the dose or by instituting treatment with calcium channel blockers such as amlodipine besylate. There is some evidence that in normotensive patients receiving cyclosporine, amlodipine therapy may prevent some of the nephrotoxicity that has been associated with this potent psoriasis treatment.79
Renal interstitial fibrosis and renal tubular atrophy are common in patients on long-term therapy with cyclosporine.80,81 Consequently, serum creatinine levels must be monitored on a regular basis. If the serum creatinine level rises more than 30% above baseline (or more than 25%, according to the United States package insert), the dosage may have to be reduced.78
Organ transplant patients taking cyclosporine, as well as other immunosuppressive drugs, to prevent rejection have experienced an increase in lymphoproliferative diseases and skin can-cers.82,83 It is hoped that the lower doses and intermittent usage of cyclosporine in psoriasis patients will not be associated with an increase in malignancies, but caution must be exercised. In one study, no increase in lymphoproliferative disorders was found in rheumatoid arthritis patients who were treated with cy-closporine for a short period (median, 1.6 years), compared with a parallel group of rheumatoid patients who were not treated with cyclosporine.84 Nevertheless, caution must be used with this powerful new psoriasis treatment.
Tacrolimus
Although tacrolimus does not have FDA approval for use in psoriasis, it is a potent immunosuppressive agent that may be substituted for cyclosporine in patients who cannot tolerate the hypertrichosis associated with this agent. Tacrolimus has proved to be effective in the treatment of psoriasis. In a double-blind trial, 50 patients with severe recalcitrant psoriasis were given either oral tacrolimus or placebo.85 In the tacrolimus group, starting dosages were 0.5 mg/kg/day, and the dosages could be increased to 0.10 mg/kg at week 3 or 6 if patient response was judged to be insufficient. After 9 weeks of treatment, patients receiving tacrolimus had an 84% reduction in Psoriasis Area and Severity Index (PASI) scores.
As with cyclosporine, there are concerns about hypertension, nephrotoxicity, and immunosuppressive effects with tacrolimus. This drug is not associated with hypertrichosis or gingival hy-perplasia. Tacrolimus has not been studied as extensively as cy-closporine for the treatment of psoriasis, and further investigations are warranted for this very effective antipsoriatic agent.
Hydroxyurea
Hydroxyurea may be considered for the treatment of psoriasis in patients with hepatic disease, because hepatotoxicity is uncommon with this agent.86 Response is slower and less complete than with methotrexate, however, and resistance to hydroxyurea may develop more frequently. Hydroxyurea is administered orally at a dosage of 1 to 2 g/day. Careful monitoring of blood counts is necessary during therapy.
Sulfasalazine
Sulfasalazine does not have FDA approval for the treatment of psoriasis but is highly effective in selected patients. It is typically given in dosages of 3 to 4 g daily. In one study, over 25% of patients given sulfasalazine stopped the treatment because of side effects (cutaneous eruptions or nausea). In clinical practice, results have been less promising than in studies.
Combination Therapy
Combinations of various psoriasis treatments have proved to be superior in efficacy to monotherapy. Acitretin is routinely used with UVB and PUVA, a combination that allows the use of smaller doses and minimizes toxicities of both retinoid therapy and phototherapy.71,72 The combination of methotrexate and ac-itretin has been used successfully despite some concern that both drugs are hepatotoxic.88 Careful monitoring of liver enzyme levels is essential. Methotrexate and cyclosporine can be used together, and their concurrent administration in small doses can result in greater efficacy and less toxicity than that which can be achieved with higher doses of either agent used alone.89 Methotrexate has also been used very successfully in combination with UVB90 and PUVA,91 although there is some concern that methotrexate may potentiate the carcinogenic effect of PUVA.92 Because cyclosporine has been associated with skin cancers, it is not routinely used in combination with PUVA. It can be used in combination with retinoids and mycophenolate mofetil.
Other Systemic Therapies
Mycophenolate mofetil, a drug that has FDA approval for the prevention of organ transplant rejection, is highly effective for some patients with psoriasis.93 Mycophenolate mofetil is the pro-drug of mycophenolic acid, a medication that was tested for psoriasis in the 1970s.94 Although mycophenolic acid was found to be highly effective in the treatment of psoriasis, the manufacturers did not pursue FDA approval for that indication because of its side effects, which included gastrointestinal toxicity and an immunosuppressive effect that resulted in herpes zoster infections in more than 10% of treated patients.
6-Thioguanine is another anticancer chemotherapeutic agent that is highly effective for psoriasis. Unfortunately, it has been associated with bone marrow suppression in approximately 50% of patients.95 Bone marrow toxicity from 6-thioguanine can be reduced by administering the drug two to three times a week rather than daily.96
Biologic Therapies
The ability to create molecules that target specific steps in the pathogenesis of psoriasis has led to the development of biologic agents that can treat psoriasis without the nephrotoxicity associated with cyclosporine and without the bone marrow and liver toxicities associated with methotrexate. Biologic agents are im-munosuppressive, and their long-term toxicity is not known. As with other immunosuppressive agents, there is concern about the potential to predispose patients to infections or malignancies. Several biologic agents have FDA approval for use in psoriasis— namely, alefacept, efalizumab, and etanercept. Others have been approved for use in other diseases but are undergoing clinical trials for use in psoriasis—namely, adalimumab and infliximab. Still other agents, such as onercept, a TNF-a blocking agent, and anti-IL-12, are at earlier stages of development.
Alefacept Alefacept is a fusion protein consisting of LFA-3 fused to the Fc portion of human IgG1. The LFA-3 portion of the molecule attaches to its naturally occurring receptor, CD2, on the surface of a resting T cell, thereby blocking T cell activation. The Fc portion of the molecule attaches to Fc receptors on natural killer cells and macrophages, resulting in apoptosis of the bound T cell.97
Alefacept originally received FDA approval as intravenous and intramuscular formulations, but it is now available only in the intramuscular form. Alefacept is administered weekly for 12 weeks in a dose of 15 mg. In one study, by 14 weeks after the start of therapy, 21% of patients achieved PASI 75 (75% reduction in disease from baseline) and 42% of patients achieved PASI 50 (50% reduction in disease from baseline). Improvement typically progresses after the completion of treatment, with maximal disease reduction 8 weeks after a second course of therapy; in one study, 33% of patients achieved PASI 75 and 57% achieved PASI 50 by this point.98 The most striking benefit of alefacept therapy is the long duration of remission achieved in a subgroup of patients. In patients who achieved PASI 75, the median time to recurrence of psoriasis (as defined by maintenance of PASI 50) was 7 months after a single 12-week course of therapy and more than a year after two courses of therapy.99
Drawbacks of alefacept therapy include the high cost of the drug and the need for weekly CD4+ T cell counts because the drug tends to reduce the number of these cells. The onset of action of alefacept is slow, with many patients achieving maximal response weeks after completing the 12-week course. Moreover, only a proportion of patients achieve a satisfactory response.
Efalizumab Efalizumab is a humanized monoclonal antibody directed against the CD11a portion of LFA-1. Efalizumab blocks the interaction between LFA-1 and ICAM-1, an interaction that is responsible for T cell activation and trafficking of T cells into inflamed skin. After a conditioning dose of 0.7 mg/kg the first week, patients self-administer subcutaneous injections of efalizumab at a dose of 1 mg/kg weekly. In double-blind, placebo-controlled trials, 22% to 39% of patients treated with weekly efalizumab for 12 weeks achieved PASI 75,100102 and nearly 60% of patients achieved PASI 50. With longer therapy, higher proportions of patients achieve greater degrees of improvement. Like the other biologic agents, efalizumab does not cause the nephrotoxicity associated with cyclosporine or the bone marrow or liver toxicity associated with methotrexate. The drug is fairly expensive, however, and flulike symptoms may develop after the first or second injection; a serious concern is the development of psoriasis rebound (defined as a worsening of psoriasis over baseline), which occurs in up to 15% of patients. To avoid psoriasis rebound, efalizumab should not be stopped abruptly but, rather, slowly converted to alternative therapies.
Etanercept Etanercept is a recombinant fusion protein that includes the p75 TNF receptor that binds to TNF-a, blocking its interaction with cell surface receptors. Etanercept originally received FDA approval for a dosage of 25 mg administered sub-cutaneously by the patient at home twice weekly for the treatment of psoriatic arthritis. Subsequently, etanercept received approval for the treatment of psoriasis at a dosage of 50 mg administered subcutaneously twice weekly for 3 months and then once weekly. In a double-blind, placebo-controlled, four-arm trial comparing placebo with three dosage regimens, analysis after 12 weeks of treatment showed that PASI 75 was achieved in 14% of patients who received 25 mg once a week, in 34% who received 25 mg twice a week, and in 49% who received 50 mg twice a week. Response rates were even higher at 24 weeks of therapy.
The drawbacks of etanercept include its cost and the need to self-inject the medication on a long-term basis. Injection-site reactions, although common, are almost always minor and seldom require any treatment other than temporarily using a different site for injections. There is evidence that TNF-a blockers can cause an exacerbation of multiple sclerosis, so the drug should be avoided in patients with a personal or family history of de-myelinating disease. Some controversy exists as to whether TNF-a blockers exacerbate chronic heart failure, and there is concern that the immunosuppressive effects of TNF-a blockers may contribute to an increase in the development of lymphopro-liferative diseases.104 Antinuclear antibodies also develop in etan-ercept-treated patients, but they are of questionable physiologic significance.
Infliximab Infliximab is a chimeric monoclonal antibody directed against TNF-a. In the short term (12 weeks), it is the most effective treatment for psoriasis, but it does not yet have FDA approval for this indication. It is administered by slow intravenous infusion at baseline, at weeks 2 and 6, and then every 8 weeks thereafter. In a double-blind, placebo-controlled trial evaluating patients at week 10, after only three infusions, 82% of patients achieved PASI 75.105 Moreover, 55% of patients maintained PASI 50 or higher during 6 months of follow-up.
Like the other TNF-a blockers, infliximab is associated with worsening of chronic heart failure, multiple sclerosis, and lym-phoproliferative diseases. In addition, infusion reactions develop in a significant proportion of patients; these appear to be related to the development of human antichimeric antibodies. Although infusion reactions are mild in the majority of patients, they can be severe, resulting in chest pain and hypotension. Pre-treating patients with antibiotics is beneficial. TNF-a blocking plays a significant role in the control of mycobacterial infection, and an increase in reactivation of latent tuberculosis has been observed in patients treated with infliximab. Consequently, patients should undergo tuberculosis testing before starting on this medication.106
Adalimumab Adalimumab is a fully human monoclonal antibody against TNF-a. It has FDA approval for the treatment of rheumatoid arthritis and has been successfully tested for psoriasis.107 Like the other biologics, adalimumab is not toxic to kidneys, liver, or bone marrow; however, also like the other biologic agents, it is quite expensive. The same concerns about heart failure, multiple sclerosis, and lymphoproliferative diseases that exist with etanercept and infliximab are also described in adalimumab’s package insert. In a three-arm, placebo-controlled trial, PASI 75 was achieved by 53% of patients who received adalimumab every other week and by 80% of patients who received it weekly. An even greater number of patients achieved PASI 50. Adalimumab, 40 mg, is given by subcutaneous injection.
Prognosis
Psoriasis is usually lifelong, but the severity of the disease may vary, with periodic exacerbations and relative remissions in some patients. Although pustular psoriasis and erythrodermic psoriasis can be life-threatening, even stable plaque psoriasis can have a negative impact on overall health, possibly because of co-morbid conditions such as psoriatic arthritis or obesity or because of complications of therapy.
Severe exacerbation of psoriasis taxes the ingenuity of even the most skilled clinician. Fortunately, because of the wide range of psoriasis therapies now available, clinicians are able to successfully treat almost all patients with psoriasis. The goal of therapy must be to minimize toxicity while achieving satisfactory improvement both in physical signs and symptoms and in patients’ quality of life.