Pathogenesis
Both acute and chronic forms of Chagas disease are recognized. The acute form, which develops soon after infection, principally affects children in endemic areas.104 Within several days of infection, an indurated erythematous lesion termed a chago-ma appears at the inoculation site. When the inoculation site is the conjunctiva, unilateral periorbital edema develops, called the Romana sign [see Figure 20]. After about 2 weeks, try-panosomes appear in the blood and invade cells, generally those of mesenchymal origin, where they multiply as intracellular amastigote forms. They are even able to proliferate in macro-phages, unless the macrophages have been activated by interfer-on gamma. The resultant intracellular pseudocysts rupture, releasing both trypanosomal and amastigote forms. Both of these forms are infectious to mammalian cells. A combination of humoral and cell-mediated immunity controls high-level para-sitemia, but despite this relative immunity, the host remains parasitemic at low levels for life. The reasons T. cruzi is able to establish lifelong infection are incompletely understood, but contributors include the following: evasion of complement-mediated cytolysis, intracellular growth in phagocytes, and the display of thousands of antigenically distinct surface proteins that appear to disrupt an effective cell-mediated immune response. Only 10% to 30% of infected persons develop chronic forms of Chagas disease. The reasons some persons develop disease and others don’t is poorly understood and may involve the initial parasite burden, continuous inflammation in critical areas, induction of autoimmunity by the chronic infection, or some combination thereof.
Diagnosis
Clinical features During the acute phase, the patient may experience intermittent or continuous fever, malaise, an evanescent rubelliform or petechial rash, hepatosplenomegaly, lym-phadenopathy, nonpitting edema of the face or extremities, tender subcutaneous nodules termed hematogenous chagomas, and, in infants, diarrhea. In severe cases, fatal myocarditis or meningoencephalitis can develop. The acute phase is usually self-limited: patients eventually become asymptomatic, and parasites can no longer be detected in the bloodstream except by PCR or by feeding reduviid bugs on the patient’s blood and examining them for infection later.
The chronic form of Chagas disease may become manifest either after an acute infection or, more commonly, after a clinically inapparent infection. It usually arises in the second or third decade of life and progresses over subsequent decades. Chronic complications of Chagas disease result from the destruction of autonomic ganglia and from myositis; the pathogenesis of these lesions is not understood.
Figure 19 Triatoma infestans, commonly known as assassin bugs or kissing bugs, are vectors for Chagas disease.
The organ most frequently involved is the heart,104 which develops biventricular hypertrophy and a mononuclear cell infiltrative myocarditis. Conduction disorders often include right bundle branch block, partial or complete atri-oventricular block, and premature ventricular contractions. Sudden death has occurred in patients with Chagas disease, and fatalities have also resulted from complications of heart failure.
The GI tract is the second most frequently involved organ system.104 The disease causes denervation leading to impaired motility and dilatation, which results in megaesophagus and megacolon. Neurologic disease is the third most frequently observed complication of chronic Chagas disease and manifests primarily as peripheral neuropathies.
Congenital infections are usually responsible for premature births. Such premature infants may have hepatosplenomegaly, abdominal distention, cardiomegaly, megaesophagus, and meningoencephalitis.
In HIV-infected patients, reactivation of Chagas disease can produce cerebral masses and, in patients with acute infections, necrotizing encephalitis. These CNS infections cannot be distinguished radiographically from toxoplasmosis, and biopsy must be performed in patients with risk factors for both infections.
Figure 20 Periorbital edema of the right eye (Romana sign) is evident in a child from Panama with acute Chagas disease.
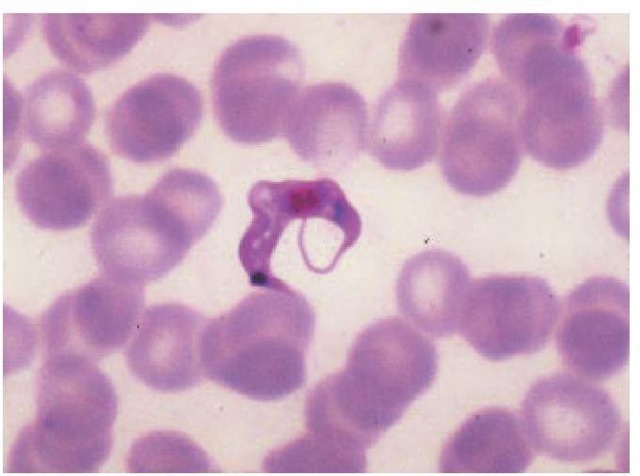
Laboratory findings In acute Chagas disease, the total leukocyte count often exceeds 18,000 cells/mm3 (70% to 90% lymphocytes), and parasites are often demonstrable in the blood or in specimens from bone marrow, lymph nodes, CSF, pericar-dial fluid, or other involved areas. On unstained blood smears, motile trypanosomes may be seen; on Giemsa-stained smears, the organisms appear as C-shaped forms [see Figure 21]. If smears do not reveal the organisms, trypanosomes may be found in stained sediment obtained by centrifuging several mil-liliters of blood after lysing the erythrocytes. Organisms may be cultured from blood on NNN medium or in blood broth. Alternatively, blood may be injected into a laboratory rodent, whose blood is then monitored for evidence of parasitemia. Xenodiag-nosis, which is not readily available, is one of the most sensitive diagnostic techniques to detect parasites. In this procedure, laboratory-reared reduviid insects are allowed to feed on a patient. If the blood ingested by the insects contains trypanosomes, the insects will become infected, and such infection can be detected by subsequent examinations of the insects’ feces for excreted parasites. PCR to detect circulating parasites is becoming available in clinical labs, is as sensitive as xenodiagnosis, and is easier to implement than xenodiagnosis. Blood containing trypanosomes is infectious and should be handled with care.
In chronic Chagas disease, a chest x-ray may reveal biventric-ular cardiomegaly and congestive heart failure. Electrocardio-graphic abnormalities are commonly seen, particularly right bundle branch block. Barium swallow or enema exams may demonstrate megaesophagus or megacolon disease. Methods other than PCR or xenodiagnosis are rarely capable of detecting organisms in the blood. Similarly, it is difficult to demonstrate parasites in affected tissues; commonly, only mononuclear inflammatory cells or fibrosis is seen in pathologic specimens. Indirect fluorescent antibody and enzyme immunoassay T. cruzi serology tests are performed by the CDC. Both of these tests rely on crude antigens derived from cultured insect forms of T. cruzi, and individuals infected with Leishmania may have cross-reactive antibodies that give a false positive result on these tests. Although these serology tests may be positive, their results may only reflect past infection and fail to establish a link between clinical findings and active Chagas disease.
Figure 21 A trypanosomal form of Trypanosoma cruzi is visible on this Giemsa-stained blood smear.
Treatment
Optimal therapy for T. cruzi infections remains to be established. Nifurtimox (available from the CDC Drug Service) eliminates parasitemia [see Sidebar, Protozoan Infection Information on the Internet]. It should be administered to patients with acute disease and to patients with chronic disease and demonstrated parasitemia.10 Side effects are frequent and include hemolytic anemia in patients with G6PD deficiency, peripheral neuritis, and psychosis. Anecdotal evidence suggests that interferon gamma combined with nifurtimox may shorten the duration of acute disease. Some authors believe that benznidazole is the drug of choice for treating T. cruzi infection, but it is not available in the United States and has a variety of toxicities, including granulocytopenia, rash, and peripheral neuropathy.104 Itracona-zole and other azoles have activity in blocking the ergosterol synthesis of T. cruzi, and itraconazole has some efficacy against chronic disease.107 Though some small studies have shown benefit of benznidazole or nifurtimox treatment of early chronic Cha-gas cardiac disease, most experts agree that the cardiovascular and GI complications of chronic Chagas disease should be managed medically. Surgical treatment may be required for mega-colon, and balloon dilatation of the lower esophageal sphincter may be needed for megaesophagus. If cardiac transplantation is contemplated, preparations to provide antitrypanosomal therapy should be made because reactivation of latent parasitemia can occur as a complication of the immunosuppressive drugs.
African Trypanosomiasis
Etiology and Epidemiology
African trypanosomiasis, or sleeping sickness, is an acute or chronic parasitic disease caused by protozoan hemoflagellates of two Trypanosoma brucei subspecies. The disease is prevalent in a broad periequatorial belt across Africa. Two forms occur in humans: West African and East African, or Rhodesian, sleeping sickness. West African trypanosomiasis is present in the tropical forests of West and Central Africa and is caused by T. brucei gambiense, a parasite not carried in any major animal reservoir. In contrast, T. brucei rhodesiense, which produces East African sleeping sickness, is prevalent in the savanna and woodlands of tropical East Africa and exists in wild animal reservoirs. Visitors to game parks are at risk for acquiring East African trypanoso-miasis.108 Both types of African trypanosomiasis are transmitted by species of tsetse flies (genus Glossina), with the riverine G. pal-palis group transmitting T. brucei gambiense and the savanna G. morsitans group transmitting T. brucei rhodesiense. T. brucei species are able to exist as chronic infections in the bloodstream and, later, in the CNS, partly because of their ability to undergo sequential antigenic variation of the major variant surface glyco-protein (VSG) that covers the trypanosome. As antibodies develop to a given VSG, most of the trypanosomes are eliminated from the circulation, but variants expressing an antigenically distinct VSG grow out and continue the infection. The combination of thousands of genes and pseudogenes for VSGs, plus an ability to create new VSG genes by recombination, allows the trypanosome to stay ahead of the immune response.
Diagnosis
Clinical features Within a few days to a couple of weeks after inoculation of organisms by a tsetse fly, a trypanosomal chancre may develop at the site of the insect bite, which is usually on exposed skin.109 The chancre initially appears as a papule and, within 2 weeks, evolves into an inflamed, painful nodule that subsequently resolves spontaneously. Trypanosomal chancres commonly occur in non-African patients but usually do not develop in African patients.
During the next phase of African trypanosomiasis, the he-molymphatic phase, trypanosomes invade the bloodstream and lymph nodes. In Africans, the development of symptoms in this phase occurs slowly, over several months, and presenting symptoms include fever, lymphadenopathy, headache, and debility. In non-Africans, however, the onset is abrupt and early, often concomitant with the development of the chancre. In non-Africans, episodes of high fever that last 1 to 7 days and recur after afebrile periods are prominent. Associated symptoms include chills, headache, malaise, and anorexia. Soft, nontender lymphadenopathy develops more prominently with West African trypanosomiasis and may include enlargement of posterior cervical nodes (Winterbottom sign). A characteristic rash, which can be observed on light-skinned individuals, occurs about 6 to 8 weeks after infection and may appear as evanescent, circinate, erythematous patches, usually located on the trunk.
The next phase in the evolution of African trypanosomiasis is CNS invasion leading to diffuse meningoencephalitis or meningomyelitis. In West African trypanosomiasis, which is a slowly evolving illness, the symptoms of sleeping sickness may not develop until years after infection. Increasing lassitude and indifference are complicated by progressive neurologic compromise leading to coma and death by inanition or intercurrent infection. In contrast, the pace of East African trypanosomiasis is much more rapid, and CNS involvement may develop earlier. Even before CNS involvement, such manifestations as somnolence, personality changes, and an inability to concentrate may appear. Pancarditis often complicates acute East African try-panosomiasis and may cause death before the onset of CNS disease. Because East African trypanosomiasis may be acquired by visitors to game preserves and is an acute febrile illness, it may be mistaken for malaria and must be considered in the differential diagnosis of a febrile patient returning from an endemic area.77
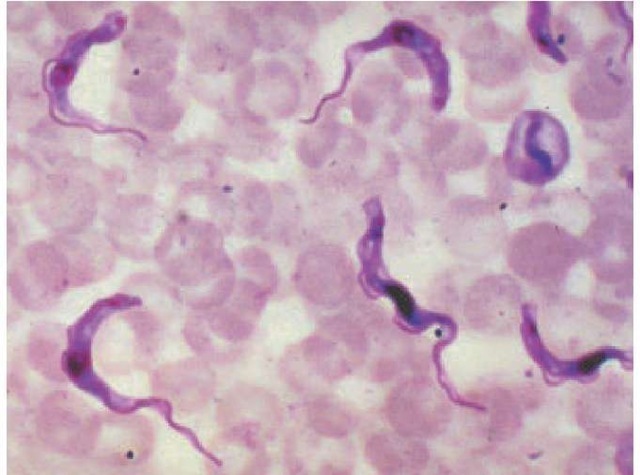
Laboratory findings The total leukocyte count is usually normal, but the differential may show mononucleosis of 50% to 70%. Serum IgM levels rise 1 to 2 weeks after parasites appear in the blood and may shoot up to more than seven times the normal level. The definitive diagnosis is made by detecting try-panosomal organisms in blood, bone marrow, fluid from enlarged lymph nodes, or centrifuged CSF [see Figure 22]. Try-panosomes may be seen moving rapidly on wet mounts of blood or other aspirates. Organisms stained with Wright, Giem-sa, or Leishman stain may be found in a chancre 48 hours before they appear in the blood. If examination of peripheral blood is unrevealing, trypanosomes may be found in fluid aspirated from involved lymph nodes or among buffy coat cells. Even in cases in which neurologic symptoms are absent, CNS disease must be excluded by performing a lumbar puncture. Even if try-panosomes are not seen in centrifuged CSF, CNS involvement may still exist, as indicated by elevations of the cell count or of protein or IgM concentrations. Serologic tests for African try-panosomiasis exist, but treatment is generally reserved for para-sitologically confirmed cases because the therapy is so toxic.
Figure 22 Trypanosoma brucei parasites, the cause of African sleeping sickness, are evident on this Giemsa-stained blood smear.
Treatment
Eflornithine is effective therapy for T. brucei gambiense infec-tions,10,109 even those refractory to the arsenical agent melarso-prol. Eflornithine is the treatment of choice for both the early he-molymphatic and the later CNS stages of West African try-panosomiasis. Because eflornithine can cause anemia, leuko-penia, and thrombocytopenia, blood cell counts should be monitored twice a week during therapy. Eflornithine has not been effective with T. brucei rhodesiense infections. The early stage of East African trypanosomiasis is treated with suramin, and the CNS stage is treated with melarsoprol.10 Both of these drugs are available from the CDC Drug Service [see Sidebar, Protozoan Infection Information on the Internet] but have a variety of toxicities; notably, melarsoprol treatment is itself fatal in 4% to 6% of patients.