The Characteristics of the Immune System
The immune system mediates the individual’s relationship with the microbial environment. Immunity involves innate, or natural, responses and highly specific acquired, or adaptive, responses. The essential difference between the two types of immunity lies in the means by which microorganisms are recognized. In innate immunity, glycolipids and macromolecules with repeat patterns that are unique to infectious organisms are recognized by cell surface receptors on macrophages, dendritic cells, natural killer (NK) and NK T (NKT) cells, as well as by the complement system. In acquired immunity, lymphocytes use very specific antigen receptors to recognize infectious agents and other antigens, either directly or when processed by antigen-presenting cells (APCs), such as dendritic cells. Thus, an interplay exists between innate and acquired immunity at the level of the APC. Once an otherwise healthy person has had an infection with bacteria or with a virus, the immune system recognizes that pathogen and prevents its recurrence. In addition, the immune system has the remarkable capacity to discriminate between antigens, even if their structures are closely related. Lymphocytes can also react to self-antigens, causing autoimmunity.
The immune response needs to be able to distinguish between self and nonself. Otherwise, T cells and antibodies would constantly be attacking autologous cells, tissue components, or even commensal bacteria. In the 1950s, Sir Frank Macfarlane Burnet first proposed that in the prenatal state, the interaction of self-antigens with antigen-specific lymphocytes leads to the elimination of self-reactive lymphocytes and hence to immunologic tol-erance.1 When immunologic tolerance breaks down, the antibodies and sensitized (antigen-reactive) cells that are directed against self-antigens cause autoimmune diseases [see 6:IX Immunologic Tolerance and Autoimmunity].
Lymphocytes
There are two major groups of lymphocytes, the T cells (also called thymus-derived lymphocytes, or T lymphocytes) and the B cells (also called bone marrow-derived lymphocytes, or B lymphocytes). T cells and B cells make up 80% to 95% of the peripheral blood lymphocytes.
T cells and B cells have a vast power of antigen recognition. Two unique features underlie this power: (1) a B cell family of variable genes, which combined can recognize an almost infinite number of antigens; and (2) a T cell family of variable genes with only a slightly more limited capacity. Neither T cells nor B cells constitute a homogeneous population of cells; each group comprises a number of subgroups that can be differentiated by the constant region of their receptors, by specific sets of develop-mentally expressed surface markers, by their location in lym-phoid organs, and by their function. The binding of combinations of monoclonal antibodies to surface receptors is currently the most specific technique used to identify the major subsets of these cells.
T Cells
Mature T cells express either a| T cell receptors (TCR-a|) or y6 TCR (TCR-yS) in a complex with the CD3 proteins. CD4 is expressed on 50% to 65% of peripheral T a| cells, and CD8 is expressed on 25% to 35% of peripheral T a| cells. Usually, T y6 have no CD4 or CD8. Although CD4 and CD8 are expressed together on cortical thymocytes, only one or the other is expressed on the complementary subsets of mature thymocytes and peripheral T a| cells (CD4+ and CD8+ T cells) [see Figure 1]. CD4+ T cells recognize antigen when the antigen is presented in association with major histocompatibility complex (MHC) class II molecules (HLA-D and HLA-DR) or in association with CD1d, the latter being NKT cells. CD8+ T cells recognize antigen in the context of MHC class I molecules (HLA-A, HLA-B, and HLA-C) [see 6:III Immune Response Mechanisms]. The context of antigen recognition by T y6 is unknown.
CD4+ helper T (TH) cells can be further differentiated into TH1 and Th2 cells on the basis of the cytokines they produce.2 Th1 cells secrete interleukin-2 (IL-2) and interferon gamma (IFN-y), which are important for cell-mediated immunity. TH2 cells secrete IL-4, IL-5, IL-6, IL-10, and IL-13, which are critical for antibody production. The cytokines that are produced by each of these cell types also influence the other cell type. For example, the IFN-y produced by TH1 cells can inhibit the function of TH2 cells, whereas IL-10, which is secreted by Th2 cells, monocytes, macrophages, and B cells, can inhibit the function of TH1 cells. In addition to having a helper function, TH1 cells can induce inflammatory cascades leading to autoimmunity, as occurs in inflammatory bowel diseases and rheumatoid arthritis.
CD4 on the surface of helper T cells plays an important role in HIV infection. In early infection, the virus uses CD4 as a corecep-tor, together with CCR5, which itself is a receptor for several chemokines, including RANTES (regulated on action, normal T cell expressed and secreted), macrophage inflammatory pro-tein-1a (MIP-1a), and MIP-1|. One percent of whites are ho-mozygous for a defect in the CCR5 receptor and are resistant to HIV infection. The chemokine receptor CCR4, which is a receptor for stromal cell-derived factor-1 (SDF-1), is involved in late HIV infection.3-5
B Cells and Plasma Cells
B cells are precursors of the immunoglobulin-producing cells (plasma cells) of the immune system and are identified by the presence of immunoglobulin on their surface. These surface membrane immunoglobulin-positive (SmIg+) cells constitute 5% to 15% of the peripheral blood lymphocytes. The majority of B cells have both IgM and IgD on their surface; about one quarter of all B cells have only IgM or IgD on their surface. One percent of B cells exhibit IgG or IgA.
On the surface of B cells is the complement receptor 2 (CR2 or CD21), which binds C3d/C3dg and Epstein-Barr virus. FcyRIIb (CD32) is the main Fc receptor on B cells, which is involved in B lymphocyte activation. A common marker that is used to identify B cells is CD19, which forms a larger complex with CD21 and CD81 (target for antiproliferative antigen-1 [TAPA-1]).6
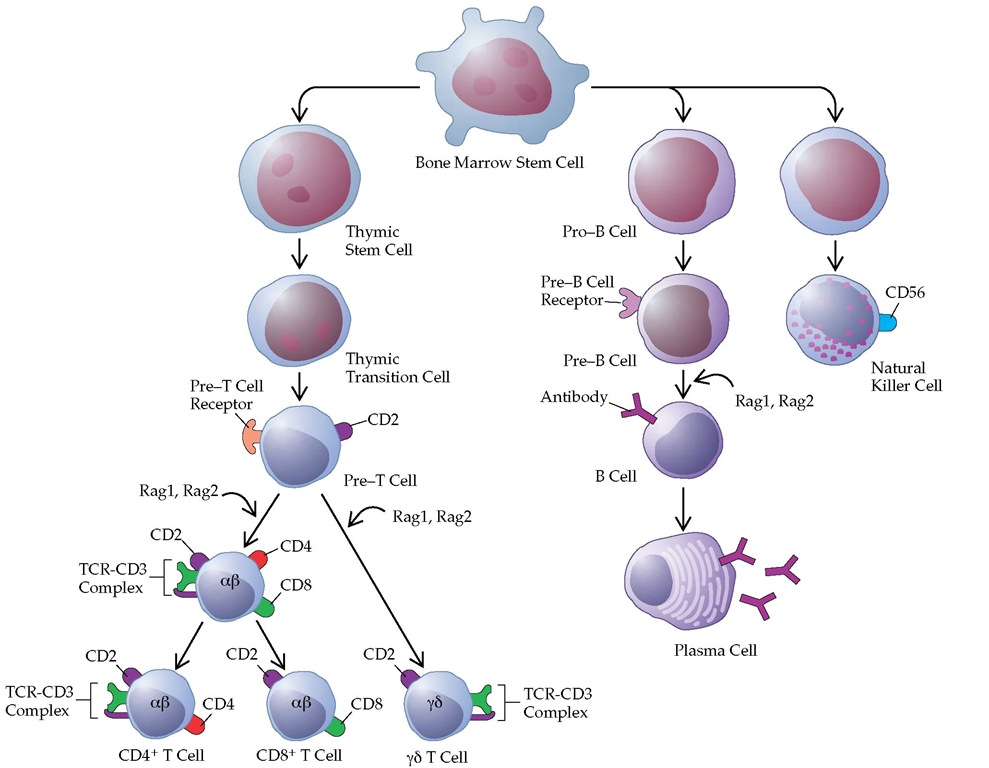
Figure 1 Lineages of cells of the immune system and of blood cells all begin with the stem cell. Stem cells that differentiate to generate B cells reside in the bone marrow, and those that produce T cells migrate from the bone marrow to the thymus. T cell maturation involves the progressive expression of selected cell surface markers and the activation of various genes, including a, p, y, and 8 genes that code for the chains that make up the ap and y8 T cell receptors (TCRs). Individuals lacking the Ragl or Rag2 enzyme do not progress past the pre-T or pre-B cell stage and therefore have no lymphocytes. Positive and negative selection of T cells occurs at the so-called double-positive (CD4+,CD8+) stage. Natural killer cells develop both in the bone marrow and in the thymus.
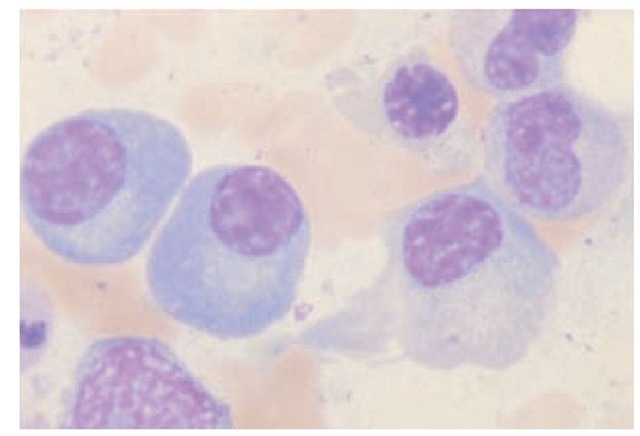
B1 cells, a subset of B cells, develop early and have a very long life. B1 cell progenitors are found in fetal liver and in embryonic omentum but not in adult bone marrow. B1 cells that express CD5 on their surface are referred to as B1a, and B1 cells that lack CD5 are called B1b. B1 cells are frequently associated with autoantibody production. They also produce substantial amounts of IL-10.7 Under the influence of antigen, T cells, and accessory cells, B cells differentiate into plasma cells, the mature antibody-producing cells [see Figure 1]. Plasma cells are larger than lymphocytes and are characterized by an eccentric round nucleus with coarse heterochromatin arranged in a cartwheel pattern. Plasma cells have a highly basophilic cytoplasm and a well-developed endo-plasmic reticulum, often organized in parallel concentric layers. Plasma cells may be distended with granular material, which consists of the antibody they are producing [see Figure 2]. Sometimes, one or more of the endoplasmic cisterns are distended by large inclusions called Russell bodies. These bodies are aggregates of incompletely formed immunoglobulin molecules. Plasma cells no longer bear surface immunoglobulin. They are also end cells, which means they do not divide. The immature precursors of plasma cells, the plasmablasts, are difficult to distinguish from lymphoblasts and large lymphocytes. Plasma cells are not normally found in the peripheral blood.
Natural Killer Cells
Natural killer cells are large granular lymphocytes that lack the TCR-CD3 complex characteristic of T cells or the SmIg characteristic of B cells. A bone marrow-derived stem cell is the precursor of T, B, and NK cells [see Figure 1]. In vitro, NK cells can kill a variety of tumor cells and virus-infected cells in a nonspecific manner; that is, they do not require previous sensitization or the presence of antibody to be cytotoxic. The granules contain pore-forming proteins that can mediate cell lysis. NK cells express killing inhibitory receptors (KIR) that recognize class 1 MHC molecules. Thus, NK cell functions are inhibited by cells that express MHC class 1 but are activated by cells lacking MHC class 1. Human NK cells express a second group of inhibitory receptors, which comprises two subunits: a variable subunit NKG and the invariant cell surface structure CD94. Its ligand is unknown. IL-12 stimulates NK cells to proliferate and to produce IFN-y, which is important for a number of immune reactions.
Figure 2 Plasma cells are the antibody-producing cells of the immune system. They differentiate from B cells; are 6 to 20 ^m in diameter; and have an eccentric nucleus, a highly basophilic cytoplasm, and a prominent, clear juxtanuclear area that contains the Golgi apparatus and the diplosome.
Monocytes, Macrophages, and Dendritic Cells
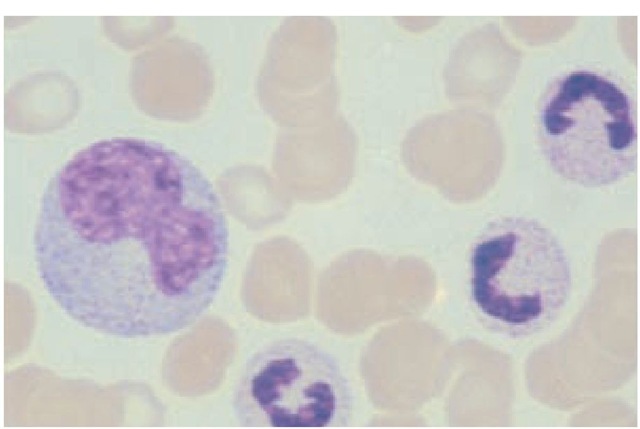
Monocytes belong to the mononuclear phagocytic system, previously called the reticuloendothelial system. They are large mononuclear cells that constitute 3% to 8% of the peripheral blood leukocytes. Their cytoplasm is much more abundant than that of the lymphocytes. Usually, their nucleus is eccentric and either oval or kidney shaped [see Figure 3]. Lysosomes filled with degradative enzymes appear as small vacuoles in the cytoplasm. Monocytes originate from promonocytes, which are rapidly dividing precursors in the bone marrow. When the mature cells enter the peripheral blood, they are called monocytes; when they leave the blood and infiltrate tissues, they undergo additional changes and are then known as macrophages. Other cells derived from this lineage include Kupffer cells, alveolar macro-phages, microglia, and osteoclasts.
Figure 3 A monocyte (large cell at left), which can reach 17 m in diameter, has abundant basophilic cytoplasm and a large eccentric nucleus.
Macrophages contain pattern recognition receptors (Toll-like receptors [TLRs] 1 through 10)10 and scavenger receptors. Furthermore, receptors for antibody and complement enhance their ability to phagocytose organisms that are coated with these substances. The antibody receptors recognize the Fc portion of IgG1, IgG3, and IgE. There are two complement receptors, CR1 and CR3. CR1 has a high affinity for the complement component C3b and a lower affinity for iC3b and C4b. CR3, also called macrophage antigen-1 (MAC-1), interacts with iC3b as well as with certain carbohydrate molecules, including carbohydrate-containing antigens of the protozoan Leishmania. Through these receptors, macrophages act as effector cells, attacking microorganisms and neoplastic cells and removing foreign material.
Of equal importance, macrophages present processed antigen to lymphocytes and thus play a major role in the induction of acquired immune responses. A small amount of MHC class II antigen is present on monocytes, and its expression is greatly increased when macrophages are activated. Macrophages can be activated by a number of cytokines, including IFN-y, granulocyte-macro-phage colony-stimulating factor (GM-CSF), macrophage-activat-ing factor (MAF), and migration inhibitory factor (MIF) [see 6:III Immune Response Mechanisms]. Cytokines such as IL-4 and transforming growth factor-| (TGF-| ) antagonize this activation.
Macrophages themselves produce a large number of soluble substances that are important in the immune response and in the process of inflammation. These substances include enzymes such as plasminogen activating factor and elastase; growth factors such as GM-CSF; cytokines such as IL-1, IL-6, IL-10, IL-12, and tumor necrosis factor-a (TNF-a); factors that are critical for combating microorganisms, such as oxygen metabolites and nitric oxide; complement components for the classical and the alternative pathway; MIPs; and factors that promote tissue repair, such as fi-broblast growth factor (FGF).
Dendritic cells are the professional APCs that engage specific responses by T cells.11-13 They are present where antigens and microorganisms have first contact with the body—for example, in the skin (Langerhans cells) and the gastrointestinal and respiratory tracts. The function of the dendritic cells is discussed [see Lymph Nodes and Spleen, below].
Lymphoid Organs and Lymphocyte Traffic
The immune system consists of a number of lymphoid organs, including the thymus, lymph nodes, spleen, and tonsils; aggregates of lymphoid tissue in nonlymphoid organs, such as Peyer patches in the gut; clusters of lymphoid cells dispersed throughout the connective and epithelial tissues of the body, as well as throughout the bone marrow and blood; and a variety of individual cells that travel from the various lymphoid organs to the rest of the body. The lymphocytes are derived from precursors in the bone marrow: the T cells develop in the thymus and are then exported to the periphery, whereas the B and NK cells develop in the bone marrow and then go out to the periphery. Of the nonlymphoid hematopoietic cells, the monocytes, macro-phages, and dendritic cells are key elements of innate and acquired immunity, whereas granulocytes (e.g., neutrophils, eosin-ophils, basophils, and mast cells) and platelets play important accessory roles in the immune system.
The Thymus
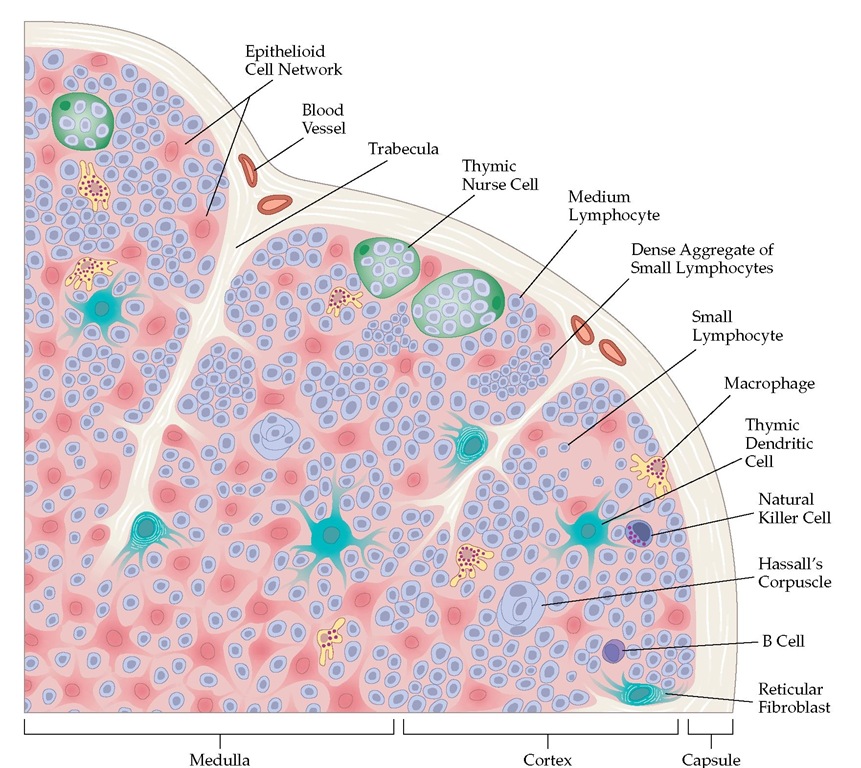
The thymus, which originates from the third and fourth pha-ryngeal pouches of the embryo, lies in the anterior mediastinum and consists of many lobules, each containing a cortex and a medulla [see Figure 4].
Figure 4 Many lobules make up the thymus gland. Most of the lymphocytes in the cortex are immature, rapidly dividing cells that can readily be destroyed by cortisone. During maturation, they move to the medulla, where they become immunocompetent and resistant to cortisone. From there, they migrate to the secondary lymphoid organs, including the lymph nodes and the spleen. Cell division and maturation are influenced by the epithelial cells; dense aggregates of these cells form bodies known as Hassall corpuscles.
Bone marrow-derived T cell precursors enter the thymus, congregating first in the subcapsular area. They develop into cells expressing the TCR-ap-CD3 complex and subsequently acquire the potential to react with different peptides bound to MHC. These cortical CD4+,CD8+ thymocytes undergo either negative or positive selection, which involves complex mechanisms [see Development of the T Cell Repertoire, below]. Only a small percentage of positively selected thymocytes, CD4+ (MCH class II recognizing) or CD8+ (MHC class 1 recognizing), migrate to the medulla and then move into the peripheral lymphoid system. It is unclear whether all y8 T cells differentiate in the thymus.
Mature T cells emigrate through the wall of the postcapillary venules of the medulla to enter the bloodstream and subsequently home to the lymphoid system’s peripheral organs.
Persons who are born without a thymus have lymphocytope-nia, with a marked depletion or an absence of T cells. The T cell zones of the peripheral lymphoid system are also devoid of lymphocytes. There is marked impairment of cell-mediated immunity, and antibody responses that require cooperation from T cells (except the IgM response) are severely impaired [see 6:VIII Deficiencies in Immunoglobulins and Cell-Mediated Immunity]. The thymus involutes with age, which may explain the development of immune system deficiencies in elderly persons.
Lymph Nodes and Spleen
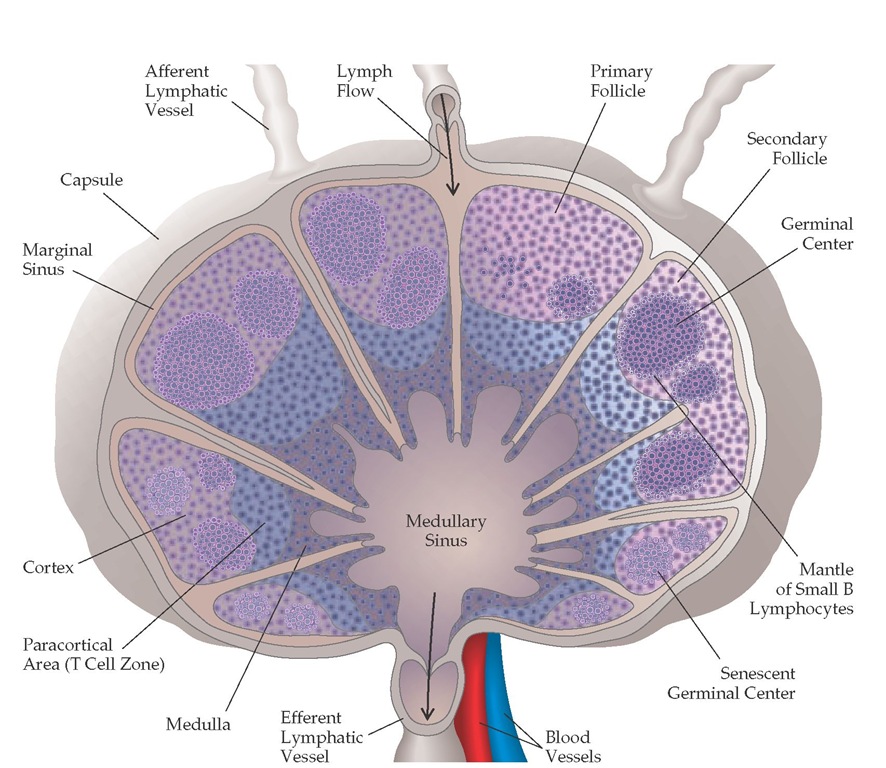
The major sites of initial T cell activation are in the lymph nodes and in the spleen, where blood-borne lymphocytes and lymph-borne antigen, soluble mediators, and cells converge [see Figure 5]. Lymph and cells enter the node in the subcapsular region, through the afferent lymphatics; percolate into the subcap-sular sinus; and leave through the efferent lymphatics in the hilum. In the spleen, the lymphocytes are concentrated in the Figure 4 Many lobules make up the thymus gland. Most of the lymphocytes in the cortex are immature, rapidly dividing cells that can readily be destroyed by cortisone. During maturation, they move to the medulla, where they become immunocompetent and resistant to cortisone. From there, they migrate to the secondary lymphoid organs, including the lymph nodes and the spleen. Cell division and maturation are influenced by the epithelial cells; dense aggregates of these cells form bodies known as Hassall corpuscles.
Figure 5 Lymph containing lymphocytes, antigen, and soluble mediators drains from surrounding tissues and enters the lymph nodes via afferent lymphatic vessels. The lymph node consists of a cortex and a medulla. The outermost area of the cortex has B cells organized into lymphoid follicles, and its deep, or paracortical, area consists mainly of T cells and dendritic cells. After B cells encounter antigens for which they have receptors, along with the appropriate T cells, central areas of marked B cell proliferation called germinal centers develop in the lymphoid follicles. As these reactions die out, the germinal centers become senescent. In the medulla are medullary cords, which are strings of macrophages and plasma cells.