Physical examination and laboratory tests
Height and weight measurements in the office are used to classify patients as overweight or obese according to BMI criteria [see Table 1]; however, these criteria may not apply to patients who have gained weight as the result of increased muscle mass from intensive exercise. Evaluation of abdominal obesity requires the use of a tape measure. A waist circumference (obtained at the level of the superior iliac crest) greater than 40 inches (102 cm) in a man or greater than 35 inches (88 cm) in a woman is considered abnormal.
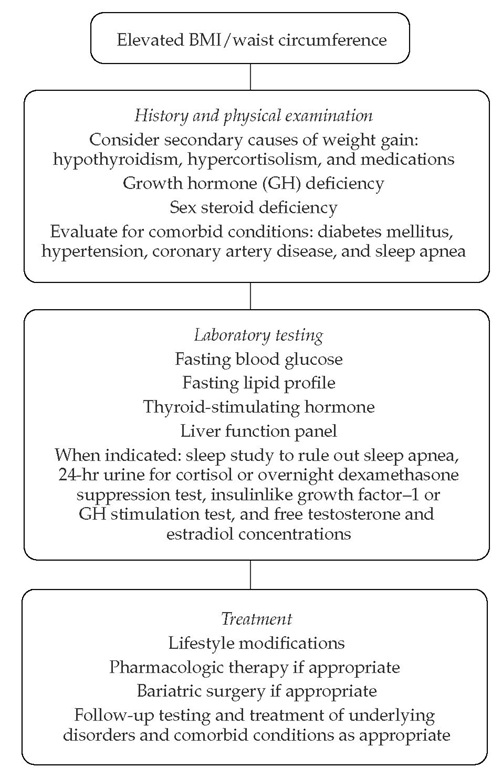
Figure 4 Evaluation, laboratory testing, and treatment of overweight and obese patients.
Specific physical findings that might indicate secondary causes of obesity include pretibial edema and delayed tendon reflexes (hypothyroidism), purple striae, supraclavicular fat pad enlargement, and muscle weakness (Cushing syndrome). Other aspects of the clinical evaluation focus on comorbid conditions. Documentation of hypertension requires properly obtained blood pressure measurements (i.e., using the correctly sized cuff for larger persons). Insulin resistance and type 2 diabetes may manifest themselves as acanthosis nigricans—patches of feathery-pigmented skin (hyperkeratotic and hyperpigmented) on the extensor surfaces of the hands and elbows, in the axilla, or on the neck [see 2:I Cutaneous Manifestations of Systemic Diseases]. Hepatomegaly can indicate hepatosteatosis, especially in centrally obese subjects.
With or without acanthosis nigricans, impaired glucose tolerance may be diagnosed by a fasting plasma glucose level between 100 and 125 mg/dl or a 2-hour glucose level between 140 and 200 mg/dl during an oral glucose tolerance test. Type 2 diabetes is diagnosed by two fasting blood glucose measurements of 126 mg/dl or greater, a 2-hour glucose level of 200 mg/dl or more during an oral glucose tolerance test, or a random glucose level of 200 mg/dl or greater and symptoms of diabetes.
Screening for macrovascular risk involves obtaining an electrocardiogram when appropriate and carefully examining the patient for xanthomata, which can indicate the presence of elevated blood levels of chylomicrons (eruptive xanthoma), type III hyperlipidemia (palmar xanthoma or tuberoeruptive xantho-ma), or familial hypercholesterolemia (tendon xanthoma). Each of these physical manifestations of hyperlipidemia, although rare in a primary care practice, indicates a severe or potentially life-threatening condition that requires urgent diagnosis and treatment. A fasting lipid profile should be obtained to complete the cardiovascular risk assessment, and if necessary, treatment should be instituted according to guidelines from the National Cholesterol Education Program Expert Panel.42 This panel also incorporated several nonlipid risk factors for cardiovascular disease into its recommendations for clinical care by defining criteria for a condition that has become known as the metabolic syndrome (also called syndrome X, the deadly quartet, and the insulin-resistance syndrome). The metabolic syndrome includes the most common abnormalities of lipid and glucose metabolism that accompany abdominal obesity [see Table 2]. Identifying these abnormalities in a patient allows the practitioner to better assign that patient’s risk for diabetes and coronary artery disease.43 Similar criteria are now recognized by the Centers for Disease Control and Prevention (CDC) as the dysmetabolic syndrome X and have been assigned a diagnosis code (277.7) in the International Classification of Diseases, 9th Revision (ICD-9). Screening laboratory tests for hepatosteotosis include a liver panel. In addition, all overweight patients should have documentation of normal thyroid function with a thyroid-stimulating hormone level.
Although obesity is associated with abnormal levels of a number of hormones and cytokines, including leptin, ghrelin, inter-leukins, and tumor necrosis factor, measurement of these variables should be limited to research protocols and are not currently recommended for general clinical practice.
|
Table 2 Criteria for Metabolic Syndrome42,151 |
|
Any Three of the Following: |
|
Increased waist circumference |
|
Men: > 102 cm (40 in) |
|
Women: > 88 cm (35 in) |
|
Fasting plasma glucose a 100 mg/dl |
|
Elevated blood pressure |
|
Systolic a 130 mm Hg Diastolic a 85 mm Hg |
|
Serum triglyceride level a 150 mg/dl |
|
Decreased high-density lipoprotein (HDL) cholesterol level |
|
Men < 40 mg/dl |
|
Women < 50 mg/dl |
Differential Diagnosis
It is important for clinicians to be alert for secondary medical causes of obesity but also to be aware that, in most cases, treatment of these coexisting diseases rarely leads to complete reversal of the obese state. As an example, hypothyroidism is relatively common in the general population and may be present in an obese patient, but the weight loss that might be expected with thyroid hormone replacement is limited and variable.
Hypercortisolemia of Cushing syndrome is a rare cause of unwanted weight gain, but clinicians should have a low threshold for screening for this disease when patients experience large amounts of weight gain in a short period, especially when the weight gain is accompanied by hypertension, diabetes, or muscle weakness. Deficiencies of growth hormone or gonadal steroids are also associated with modest increases in body adiposity. Growth hormone deficiency can lead to reduced muscle mass and increased fat mass, which is improved with hormone replacement therapy.44 Similar changes in body composition have been described in hypogonadal men45 and in postmenopausal women.18 Unfortunately, obesity is often accompanied by low levels of IGF-1 and, in men, low testosterone levels because of low sex-hormone-binding globulin levels. To distinguish obesity-associated low testosterone from a true deficiency state, free testosterone levels can be measured. In addition, weight loss will increase both IGF-1 and total testosterone levels in obese patients but not in patients with true deficiencies.
A number of medications can lead to unwanted weight gain and obesity; if possible, such patients should be switched to alternative agents [see Table 3]. Drug-related weight gain occurs most commonly during long-term glucocorticoid treatment of inflammatory conditions (e.g., asthma and inflammatory arthritis), with immunosuppression after transplantation, and with cancer chemotherapy. When possible, reducing or discontinuing a glucocorticoid in favor of an alternative medication can reverse this weight gain. Patients with type 1 or type 2 diabetes often gain weight after starting therapy; this weight gain is proportional to the degree of improved glycemic control and results from a reduction in glucosuria and improvement in metabolic efficiency.46 Long-term studies have shown that intensive insulin treatment of type 1 diabetes can result in excessive weight gain and obesity in up to 25% of patients; in type 2 diabetes, intensive glycemic control with insulin, a sulfonylurea, or one of the thiazolidinediones may also result in greater weight gain than that predicted by improved glycemic control alone.47 Therapy with metformin plus nighttime long-acting insulin may reduce or prevent this extra weight gain,48 and newer diabetes medications, such as pramlintide and exenatide, can improve glycemic control in both type 1 and type 2 diabetes with a modest weight loss.47 Neuropsychotropic drugs, particularly newer antipsychotic and antiseizure medications, have been associated with weight gain (sometimes massive), obesity, and diabetes.
Table 3 Medications Commonly Associated with Weight Gain and Obesity, with Possible Alternative Agents4749152
|
Medication Class |
Agents |
Alternatives |
|
Steroids |
Glucocorticoids |
Asthma: inhalers |
|
Cancer chemotherapy: non-glucocorticoid-based regimens |
||
|
Rheumatoid arthritis: methotrexate and remitting agents |
||
|
Antidiabetic drugs |
Insulin |
Metformin, acarbose, pramlintide, exenatide |
|
Sulfonylureas |
||
|
Thiazolidinediones |
||
|
Antiepileptic drugs |
Gabapentin |
Lamotrigine Topiramate Zonisamide |
|
Valproic acid |
||
|
Antipsychotic agents |
Clozapine |
|
|
Olanzapine |
Aripiprazole |
|
|
Quetiapine |
Haloperidol |
|
|
Risperidone Sertindole |
Ziprasidone |
|
|
Antidepressants |
Bupropion |
|
|
Tricyclic antidepressants |
Nefazodone |
|
|
Monoamine oxidase inhibitors |
Selective serotonin reuptake inhibitors |
|
|
Mirtazapine |
Venlafaxine |
Treatment
As a first step in the management of obesity, appropriate follow-up testing and treatment should be provided for any secondary causes of obesity and comorbid conditions identified during screening. Then, the approach to the treatment of obesity is similar to that of other chronic conditions, such as hypertension, hypercholesterolemia, and diabetes. Intervention starts with lifestyle measures for 3 to 6 months. For obesity, these lifestyle interventions include improved diet and increased activity. For patients whose weight does not change with lifestyle intervention alone or whose weight loss is insufficient to lower their long-term health risk, consideration is then given to phar-macologic or surgical management. An NIH expert panel has suggested that patients whose BMI is 30 or more or who have a BMI of 27 or more plus obesity-related risk factors (i.e., diabetes, hypertension, or hyperlipidemia) could be considered for phar-macologic therapy.3 Patients with a BMI of 40 or more or a BMI of 35 or more plus obesity-related risk factors could be considered for surgical therapy.
The weight-loss goal for the treatment of obesity is sustained weight loss of 5% or more of initial body weight. Although this goal does not result in attainment of a normal body weight (BMI of 19 to 25) in the majority of patients, it still represents a weight loss that can be achieved with available intervention modalities and that has been associated with lower morbidity, including reductions in risk for diabetes and heart disease.51,52
For some patients who are experiencing a period of weight gain, weight stability may be their primary goal. This is especially common in patients who have just completed a low-calorie weight-loss program and are struggling to remain below their initial weight.
Nonmedical (lifestyle) therapy
Diet Modification
Caloric restriction Hypocaloric diets have been a mainstay recommendation by the medical community for obese patients. These diets range from a moderate reduction in daily intake (200 to 500 fewer calories a day) to more stringent, very low calorie diets (600 to 800 total calories a day), which require careful follow-up by a nutritionist and a physician to avoid life-threatening electrolyte disorders and symptomatic cholelithiasis. Although it is possible to achieve short-term weight loss with these strategies, long-term weight loss is poor even when behavior-modification weight-maintenance programs are continued. Analyses of published data on long-term weight-loss maintenance showed that approximately 50% of the initial lost body weight is regained within the first 1 to 2 years, and 95% or more is regained by 5 years after the completion of the calorie-restriction phase.53,54 This restoration of lost body weight after a period of calorie-restriction-induced weight loss can be explained by the reduction of fat-dependent feedback signals to the brain, such as leptin, which then activate counterregulatory systems to restore body weight to baseline [see Pathophysiology and Pathogenesis, above].
The long-term failure to maintain weight loss after caloric restriction also indicates that the central set point for body weight is not reset at a lower body weight with the passage of time. Another important implication of these data is that obesity treatments lacking mechanisms that interfere with this counterregu-latory system will likely fail to allow long-term weight-loss maintenance. Some existing therapies do result in limited weight loss without activation of appetite (see below) and can be combined with caloric restriction for improved long-term weight-loss maintenance; these include a low-fat diet,55,56 exercise,56 and pharmaco-logic treatments.
Dietary-fat restriction An increase in dietary-fat intake leads to obesity in animal studies and has been associated with a higher prevalence of overweight and obesity in many human-population studies.25 Prospectively randomizing overweight and obese persons to ad libitum feeding (eating until one feels full, then stopping) of a fat-restricted diet results in a spontaneous reduction in caloric intake and subsequent modest weight loss, compared with results in persons on a diet that contains a higher amount of fat typical of Western societies.59 This calorie reduction occurs despite a concomitant fall in leptin levels, in contrast to the increase in appetite that follows a fall in leptin levels with weight loss from caloric restriction.59-61 By implication, an increase in dietary fat results in a state of central leptin resistance, requiring a higher level of body fat and leptin levels to attain a new body-weight equilibrium,62 whereas dietary-fat restriction leads to partial improvement in leptin signaling, resulting in spontaneous reduction in appetite and body weight.
The average amount of weight loss attributable to a low-fat diet in these studies, however, is only on the order of 3 to 4 kg (6.6 to 8.8 lb).59 In addition, the weight-loss responses of persons to a low-fat diet can vary tremendously, with some individuals losing 13 kg (28.7 lb) or more and others losing no weight or even gaining weight.63 This variable response to a lifestyle intervention, such as a change in a specific diet component, is common in chronic diseases whose expression results from an interaction between a genetic predisposition and environmental influence. In patients with hypertension, for example, blood pressure reductions in response to restriction of dietary salt are also heterogeneous.
An apparent paradox has been reported in that the average percent fat content of the American diet is dropping, yet the weight of the American population keeps increasing. Although it is true that the average percentage of total calories from fat in the American diet has declined over the past several decades (from 36% to 34%, according to the most recent NHANES data),64 this did not occur because Americans have been eating less fat (daily dietary fat intake was 81.9 g in 1972 and increased to 85.5 g in 1990) but, rather, because total calories increased, leading to a lower fat percentage.65 In contrast, studies that documented weight loss with a lower fat intake did so by lowering the absolute amount of fat in the diet. It is worth noting that the levels of fat restriction leading to weight loss in these studies were not severe. Severe fat restriction (< 20% of total calories) may not be sustainable for many patients because of limited food options and palatability.
Dietary-carbohydrate changes Increasing dietary-carbohydrate intake while lowering total fat intake results in modest spontaneous reduction in caloric intake and weight loss in overweight and obese persons. In the studies that documented this effect, the additional carbohydrates were derived from fruits, vegetables, and grain products, and the resulting increase in dietary fiber also may have played a role in greater satiety and weight loss. In society (especially in young people), however, dietary carbohydrates have increasingly been consumed in the form of processed foods sweetened with sucrose or fructose. These simple carbohydrates (especially fructose) may potentially have deleterious effects on insulin resistance, lipid levels, and body weight when consumed in large amounts.29,66
Paradoxically, severe carbohydrate restriction (< 30 g/day) may also lead to modest spontaneous weight loss without initial activation of appetite. Such severe carbohydrate restriction initially mobilizes glycogen stores in the liver and induces ketogen-esis, and the resulting diuresis accounts for some of this weight loss.67 At one time, the ability to draw meaningful conclusions about the longer-term safety and efficacy of low-carbohydrate diets was impeded by the paucity of controlled studies and the variability of carbohydrate restriction from study to study (from < 20 to a 200 g/day).68 Although methodological issues remain, randomized, controlled studies have now shown that during the first 6 months of diet treatment, persons placed on a low-carbohydrate diet lose weight more rapidly than those placed on a low-calorie, low-fat diet.69-71 Subsequently, however, individuals on the low-carbohydrate diet either stop losing weight or regain weight, and by 1 year, weight loss is the same with the two di-ets.70-72 The average 1-year weight loss ranged from 2.5 to 5.1 kg in these studies, and both diets had a high dropout rate, of approximately 30% to 40%.70-72 Contrary to popular beliefs about the potential adverse effects of consuming diets high in fat or carbohydrates, lipid levels and glucose metabolism improved with both diets in proportion to weight loss.69-73
Dietary-protein changes Increasing dietary-protein intake has also been associated with weight loss. In one of the few prospective, randomized studies of an ad libitum high-protein diet (fat restricted), obese patients experienced significantly greater weight loss than obese control subjects who followed a regular diet or a low-fat, high-carbohydrate diet over a period of 6 months.74 After 12 months, however, total weight loss on the high-protein diet was attenuated and no longer differed from that seen with the high-carbohydrate diet.74
Most nutrition societies recommend limiting protein intake to approximately 10% to 15 % of daily calories because of concerns regarding long-term health consequences of high intake of protein (especially animal protein). These concerns include the possible association of increased protein intake with intestinal cancers, bone disease, and renal disease. To date, prospective studies have shown that increasing dietary protein increases the glomerular filtration rate,75 which may be harmful to patients with existing renal disease or diabetes, but the long-term effect of increased glomerular filtration rate in otherwise healthy persons is not known.
Dietary fiber Increased dietary fiber has been shown to improve body weight and cardiovascular risk factors .76,77 Typical high-fiber foods include fruits, vegetables, oat and wheat bran, and legumes, which are also low in fat. Even after controlling for low-fat content, however, diets higher in fiber result in reduced intake and a weight loss of approximately 2 kg.76 Although it is possible to increase fiber through the use of supplements such as psyllium or methylcellulose, the current intake of fiber in the United States of about 15 g/day could be increased to 25 to 30 g/day by avoiding calorically dense, refined-sugar foods and increasing consumption of fruits, vegetables, and whole-grain products.
Summary of dietary recommendations Overall, an initial recommendation to lower dietary-fat intake and increase dietary-fiber intake for weight loss is reasonable and supported by the scientific literature. Long-term studies of greater than 1 year have not been conducted to show that this weight loss is sustained. Nevertheless, animal models of obesity, population studies, and prospective studies of 1 year or less of low-fat, high-fiber diets versus high-fat diets have documented that a high-fat diet is detrimental to body weight and that restriction of dietary fat to 25% to 30% of calories and an increase of 10 to 15 g of fiber a day result in a significant, albeit limited, weight loss for the average patient.
It is important that clinicians discourage unrealistic expectations about weight loss from a low-fat, high-fiber diet so that patients do not become disillusioned with this therapy. Also, patients should be informed that low-fat, high-fiber diets have been shown to reduce numerous health risks, especially when instituted as part of an overall lifestyle change that includes exer-cise.50,78 For this dietary advice to be effective, however, it is often necessary to refer patients to a nutritionist for evaluation and follow-up.
A low-fat diet can be achieved by substituting either carbohydrate or protein for fat; this allows tailoring of the diet to the individual patient. Some patients may respond better to a high-carbohydrate diet in terms of food preferences and weight loss, whereas others might have better responses to a high-protein diet, although all these diet variations have shown only moderate weight loss.72 A low-carbohydrate diet cannot currently be recommended for clinical practice, because it has not been shown to be superior to other diets70,72; the long-term health outcomes of sustained ketosis are uncertain; and increased intake of saturated fat and trans-fatty acids may negate the benefit of weight loss by increasing serum cholesterol and triglyceride levels in some pa-tients.72,73 Many questions concerning the effects of a higher protein intake (up to 30% of total calories) on patients’ renal function remain unanswered. For this reason, high-protein diets should be avoided in patients with existing renal disease and diabetes.