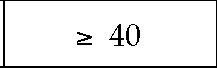Obesity and its associated disorders are leading causes of morbidity and premature mortality around the world. Obese persons are also vulnerable to low self-esteem and depression because of the psychological and social stigmata that can be associated with obesity. Despite societal prejudicial perceptions that obesity develops because of deficient self-control, research has provided insight into the physiology behind unwanted weight gain. Indeed, during the past decade, the field of body-weight regulation (the study of the homeostatic mechanisms controlling body weight and fat content and the pathophysiology leading to unwanted weight gain or weight loss) has undergone an explosion in research, particularly in the area of neuroendocrine control of appetite and energy expenditure. As with other leading diseases in developed countries, such as hypertension and diabetes, obesity is recognized as a chronic condition resulting from an interaction between environmental influences and an individual’s genetic predisposition to weight gain.
The initial evaluation of overweight and obese patients begins with the exclusion of secondary causes of weight gain and the identification of comorbid disorders such as hypertension, diabetes, heart disease, and sleep apnea. Once screening is completed, the approach to the treatment of overweight and obesity is similar to that of other chronic diseases: begin with lifestyle improvements, and then consider medical and surgical options. Although the weight loss that accompanies current therapeutic options is modest on average, the future promises better diagnostic and treatment options for obesity that are based on research into the mechanisms of weight regulation and their role in unwanted weight gain and maintenance of the obese state.
Definition of Obesity
Obesity is an abnormal accumulation of body fat in proportion to body size. Overweight persons have a body-fat proportion that is intermediate between normal and obese. Ideally, an obesity classification system would be based on a practical measurement of body fat that could be performed in the office, would accurately predict disease risk, and would apply to patients from diverse ethnic backgrounds. The most direct measures of body fat, such as underwater weighing or dual-energy x-ray absorptiometry (DXA) scanning, are impractical for use in a clinical setting. Indirect estimates of body fat are clinically more practical.
Classification of Obesity
Body mass index
Body mass index (BMI), which is calculated by dividing the body weight in kilograms by height in meters squared, is a classification system that attempts to allow comparison of weights independent of stature across populations. Except in persons who have increased lean weight as a result of intense exercise (e.g., bodybuilders), BMI does correlate with percentage of body fat, but this relationship is independently influenced by sex, age, and race.1 In the United States, data from the second National Health and Nutrition Examination Survey (NHANES II) were used to define obesity in adults as a BMI of 27.3 kg/m2 or more for women and a BMI of 27.8 kg/m2 or more for men.2 These definitions were based on the gender-specific 85th-percentile values of BMI for persons 20 to 29 years of age. In 1998, however, the National Institutes of Health (NIH) Expert Panel on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults adopted the World Health Organization (WHO) classification for overweight and obesity.3 The WHO classification, which predominantly applies to people of European ancestry, assigns an increasing risk for comorbid conditions—including hypertension, type 2 diabetes mellitus, and cardiovascular disease—to persons with higher BMIs [see Table 1] relative to persons of normal weight (i.e., those with a BMI between 18.5 kg/m2 and 25 kg/m2). Asian populations, however, are known to be at increased risk for diabetes and hypertension at lower BMI ranges than those for non-Asian groups.4 Consequently, the WHO has suggested lower cutoff points for consideration of therapeutic intervention in Asians: a BMI of 18.5 to 23 kg/m2 represents acceptable risk, 23 to 27.5 kg/m2 represents increased risk, and 27.5 kg/m2 or higher represents high risk.5
Fat distribution
In addition to an increase in total body fat, a proportionally greater amount of fat in the abdomen or trunk compared with fat in the lower extremities or hips has been associated with increased risk for diabetes, hypertension, and heart disease in both men and women.6 Abdominal obesity is commonly reported as a waist-to-hip ratio, but it is most easily quantified by a single circumferential measurement obtained at the level of the superior iliac crest.3 Current guidelines categorize men at increased relative risk for coronary artery disease, diabetes, and hypertension if they have a waist circumference greater than 40 inches (102 cm); women are at increased risk if their waist circumference exceeds 35 inches (88 cm) [see Table 1]. Thus, an overweight person with abnormal fat patterning may be at high risk for these diseases even if that person is not obese by BMI criteria. In those of Asian descent, abdominal (central) obesity is recognized to be a better predictor of comorbidity than BMI.7 Therefore, the WHO has recommended lower waist circumference cutoffs to assign increased risk for comorbidities in this population: 36 inches (90 cm) or more in men and 32 inches (80 cm) or more in women.
Table 1 Classification of Weight and Risk for Comorbid Conditions2
Epidemiology
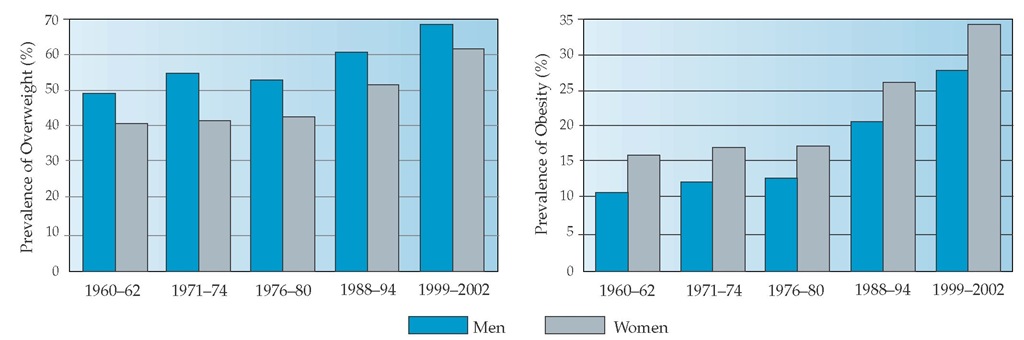
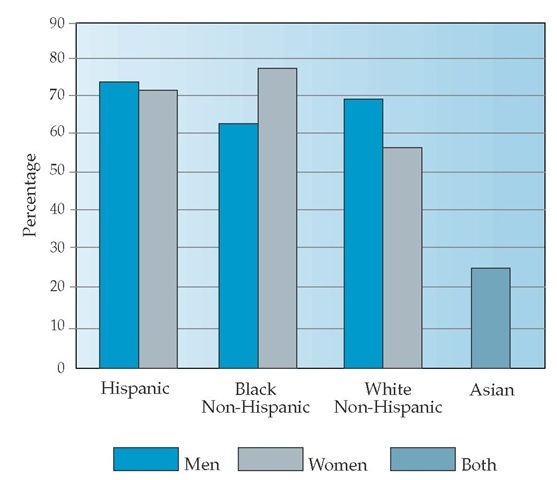
In the United States, the prevalence of overweight has been increasing over the past several decades [see Figure 1]. In the most recently published United States data (1999 to 2002), 65% of adults are overweight (BMI 25 to 30 kg/m2), 30% of the total population are obese (BMI 30 to 40 kg/m2), and 5% have a BMI of 40 kg/m2 or higher.8,9 The prevalence of obesity has also risen in some minority populations, with the highest rates found in some Native American groups, Hispanics, and African Americans; the lowest rates have been found in populations of Asian ancestry [see Figure 2].9-12 Prevalence rates for obesity in the United States are also highest in populations with less education and lower income levels.12 Internationally, obesity rates are generally lower than those in the United States.13 However, even in societies that traditionally had the lowest prevalence of overweight and obesity, the rates of weight gain are beginning to meet or exceed those of Western societies.
The age at which obesity is most prevalent has also increased. Until NHANES III (1988 through 1994), obesity in the United States peaked between the ages of 40 and 59 years, then declined in the older-age groups.9 According to the most recent NHANES data (1999 to 2002), the prevalence of obesity now remains high past the age of 60 years, reaching 30.5% in men and 34.7% in women.9 In studies that have measured body composition in unselected populations, fat mass also peaks just past middle age in men and women, but percent body fat continues to increase past this age, particularly in men, because of a proportionally greater loss in lean mass.15-17 The menopausal period has also been associated with an increase in percent body fat and propensity for central fat distribution, even though total body weight changes very little during this time.18 A propensity for greater abdominal adiposity has also been demonstrated in men,19 in older individuals,20 and in persons with impaired glucose tolerance or type 2 diabetes.21
Etiology and Genetics
Studies of populations, families, adoptions, and twins have established a strong genetic role in determining body weight. Estimates of the genetic contribution to the variance of relative body weight and adiposity range from a low of approximately 20% to a high of 90%.22 The largest study to date to address the contribution of nature versus nurture to body weight, which used a dataset that included over 25,000 twin pairs and 50,000 biologic and adoptive family members, found that genetic factors accounted for 67% of the variance in adiposity in men and women.22 Rarely, childhood-onset obesity will manifest itself as a result of a single-gene obesity syndrome, such as Prader-Willi syndrome or Bartlett-Biedel syndrome, or from a mutation in one of the genes encoding proteins involved with body-weight regulation, such as the pro-opiomelanocortin (POMC) gene, which makes a-melanocortin-stimulating hormone (a-MSH); the melanocortin receptor (MC4); leptin; the leptin receptor; and prohormone convertase enzymes.23
Although the genetics explaining the tendency toward overweight and obesity in the majority of the population remains to be elucidated, over 600 genetic markers have been described in association with obesity-related variables in humans (e.g., BMI, skin-fold thickness, waist-to-hip ratio, fat mass, and percent fat mass).23 With time, discoveries of specific gene products, the role that these proteins play in the pathophysiology of weight regulation, and their interaction with the environment in the expression of unwanted weight gain should lead to more specific pharmacologic treatments for overweight and obese patients who fail to respond adequately to lifestyle measures alone.
Epidemiologic studies have identified several environmental factors that contribute to the continued weight gain documented over the past several decades in westernized countries. The foremost among these factors are an increasingly sedentary lifestyle (e.g., increased car use, community and work environments that discourage activity, and more time spent watching television) and the availability of energy-dense (high-fat, concentrated-sugar), low-fiber foods.24-28 In children, the increased consumption of sugar-added beverages and reduction of dairy intake have also been associated with greater weight gain in prospective stud-ies.29,30 Similar environmental predictors of weight gain have been described in societies adopting Western lifestyles in the transition to First World economies.14,31 Additional societal trends that are thought to have contributed to the increasing weight gain in the United States include smoking cessation (cigarette smoking is known to reduce body weight)25,32 and eating a greater proportion of food away from home, particularly at fast-food restaurants, where food is typically very calorically dense.
Figure 1 Time trends of age-adjusted prevalence of overweight (BMI a 25 kg/m2) and obesity (BMI a 30 kg/m2) in United States men and women who are 20 years of age and older.
Figure 2 Age-adjusted percentage of United States adults who were overweight (BMI a 25 kg/m2), by sex and race/ethnicity, 1999-2002.8 Data for Asians include both men and women in 2003.
Pathophysiology and Pathogenesis
Arguably the most significant recent advances in the science of obesity have been in the area of neuroendocrine control of energy homeostasis, including the understanding of the mechanisms that lead to unwanted weight gain and the counterregula-tory systems that restore weight lost after caloric restriction. At its most basic level, body weight is the end result of a balance between energy taken in and energy expended. Weight gain ensues when more energy is consumed than expended. Weight loss occurs through restriction of energy intake, increased energy output, or both. However, this simple model fails to incorporate what are now known to be complex homeostatic systems that counteract voluntary energy perturbations, whether they be forced overfeeding or caloric restriction.
A homeostatic model of weight regulation is conceptually identical to other tightly regulated systems in the body. For example, blood glucose levels reflect input from meals and hepatic stores balanced against clearance through uptake by peripheral tissues and excretion by the kidneys. Glucose levels are kept within a normal range by complex, integrated responses from insulin, glucagon, and other so-called counterregulatory hormones such as catecholamines, cortisol, and growth hormone, which regulate production and clearance. Elevated blood glucose levels and diabetes result when the secretion of primary regulators (i.e., insulin and glucagon) is impaired, when resistance to insulin signaling develops, or both.
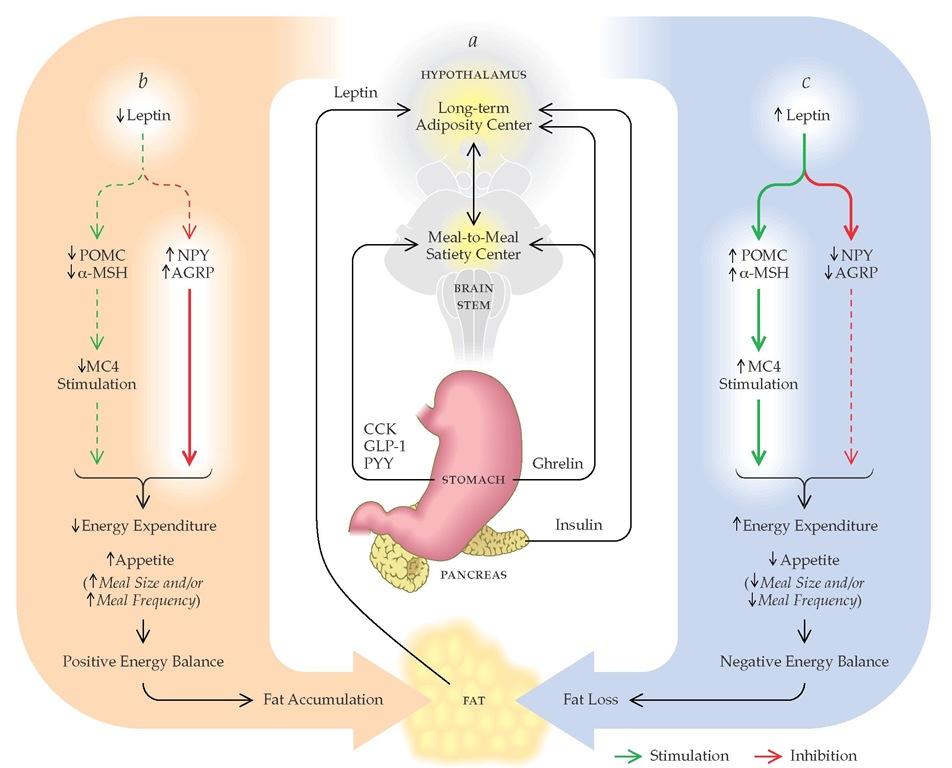
Like glucose, body weight is regulated at multiple levels to maintain a normal range or set point through an interaction between systems that control meal-to-meal intake (satiety) and those that control relative fat mass (adiposity) [see Figure 3].M Although short-term (meal-to-meal) signals such as cholecys-tokinin have been studied for decades, a long-term afferent signal from the fat tissue, leptin, was not discovered until 1994.35 Leptin is a hormone that is secreted by fat cells in direct proportion to total fat mass, is transported across the blood-brain barrier, and has receptors in hypothalamic nuclei that control appetite and energy expenditure.34 When leptin levels decline with weight loss from caloric restriction or when they increase with overfeeding, altered signaling in central hypothalamic centers become integrated with other input signals (e.g., insulin and ghrelin) to set in motion systems that restore body weight to baseline. Therefore, most obese patients fail to sustain long-term weight loss with calorie restriction alone because of activation of these counterregulatory systems and their promotion of a positive energy balance. In this feedback-loop model of weight regulation, primary obesity results when leptin signaling to central centers is reduced (leptin resistance),34 resulting in uncompensat-ed weight gain that eventually reestablishes energy homeostasis at a higher body-weight set point and blood level of leptin, analogous to insulin levels rising in compensation for acquired insulin resistance.
Although this model oversimplifies the complex nature of body-weight regulation, it nonetheless provides a starting point for clinicians in their education of patients about the pathophys-iology of obesity and in the rationale for medical management. To achieve sustained weight reduction in overweight and obese patients, interventions must prevent activation of counterregula-tory systems that act to restore lost weight by increasing appetite or reducing energy expenditure. Future medical therapies will be based on an understanding of the body’s weight regulatory system and will have greater promise for success in maintaining weight loss.
With aging, dysregulation of a number of hypothalamic-pitu-itary systems may contribute to increased fat mass and sarcope-nia. For example, growth hormone secretion diminishes with age.36 Prospective trials in older adults that involved replacing growth hormone and targeting levels of insulinlike growth fac-tor-1 (IGF-1) to the midnormal to upper-normal range have demonstrated improved body composition (less fat, more lean tissue) and, in some studies, reduced central fat.36-38 In addition, the decline in testosterone levels in men, the drop in estrogen levels in women at menopause, and increased levels of cortisol in both sexes may also contribute to reduced muscle mass, central fat distribution, or both.
Diagnosis
The history, physical examination, and laboratory evaluation of overweight and obese patients are directed toward three goals: first, to identify secondary causes of obesity [see Differential Diagnosis, below]; second, to identify comorbid conditions [see Figure 4]; and third, to establish the patient’s dietary and activity habits.
History
A number of the symptoms associated with diseases that can cause or contribute to unwanted weight gain, such as hypothy-roidism or Cushing disease, occur frequently in overweight patients. These include fatigue, aches, cold intolerance, constipation, poor exercise tolerance, central obesity, loss of libido, and depression. Deciding when to screen a patient for secondary causes of obesity, therefore, can be a challenge for the practitioner. Establishing a pattern of weight gain may be helpful. A patient with a lifelong history of being heavy and a stable adult weight is unlikely to have a secondary cause of obesity. A sudden or rapid weight gain over a few months or years, however, especially when accompanied by onset of comorbid conditions, may correspond to the prescription of medications that contribute to excess weight gain (especially steroids and newer an- tipsychotics) or indicate onset of an illness that requires further evaluation.
Figure 3 (a) A feedback model for body-weight regulation in humans based on data from animal models.34 Hypothalamic centers that control long-term energy homeostasis sense fat stores through circulating levels of leptin and insulin. Satiety signals (short-term or meal-to-meal regulators) from the gut are relayed through the brain stem to the hypothalamus, where they are integrated with signals reflecting fat stores. These integrated signals then affect appetite and energy expenditure so as to maintain body weight within a set point range. (b) Leptin controls appetite and energy expenditure in the hypothalamus by alternatively stimulating production of pro-opiomelanocortin (POMC) and a-melanocortin (a-MSH) and inhibiting production of neuropeptide-Y (NPY) and agouti-related protein (AGRP). a-MSH binds to the melanocortin-4 receptor (MC4), which inhibits appetite and increases energy expenditure. NPY and AGRP stimulate appetite while decreasing energy expenditure. Reduced leptin secretion, such as that which occurs after voluntary caloric restriction,59146 leads to enhanced NPY/AGRP signaling, diminished MC4 signaling, and positive energy balance once caloric restriction ceases.147148 On the other hand, overfeeding leads to increased fat mass and leptin secretion,149 reduced NPY/AGRP signaling, increased MC4 signaling, and negative energy balance148,150 until body weight is restored to baseline. (CCK—cholecystokinin; GLP-1—glucagonlike peptide-1; PYY—peptide YY)
The history should include questions about diseases for which overweight and obese patients are at higher risk, including hypertension, impaired glucose tolerance or diabetes, hyperlipi-demia, heart disease, pulmonary disease, and sleep apnea. These conditions may cause minimal or no symptoms and therefore may be present for months or years before a diagnosis is made. Sleep apnea in particular is a common cause of fatigue and poor concentration or work performance in obese patients; these symptoms are often mistakenly ascribed to an abnormally functioning thyroid gland (despite normal results on thyroid function tests) or a so-called altered metabolism. This diagnosis may be missed unless the clinician specifically asks about characteristic symptoms: restless sleep at night, snoring or observed apnea, fatigue or headache upon awakening and during the daytime, and spontaneous daytime sleep when inactive or while driving. In severely obese patients, increasing peripheral edema, orthopnea, and worsening exercise tolerance may be symptoms of congestive heart failure or pulmonary hypertension and right-sided heart failure from severe sleep apnea. New-onset headaches may indicate normal-pressure hydrocephalus. Gastroesophageal reflux disease usually results in heartburn or an acid taste in the throat. During a period of weight gain, women may develop irregular periods or symptoms of androgen excess. Although commonly diagnosed as polycystic ovary syndrome (PCOS), these findings differ from classic PCOS in that they occur after menar-che and are not usually associated with polycystic ovaries.
Finally, inquiring about past and present dietary and activity habits is important for subsequent discussions of medical and surgical management. Most overweight and obese patients will have made numerous attempts to lose weight, through diets, exercise regimens, or commercial weight-loss programs. Because of unrealistic expectations and the inevitable weight regain that occurs, patients are often discouraged or leery of new advice. These failures can also compound feelings of guilt or inadequacy, fueling cycles of worsening self-image and depression. A broad survey of the types of foods people eat can often be accomplished in an office visit; in particular, asking about intake of calorically dense foods, including sodas, and frequency of meals outside the home may identify habits that can be improved. More detailed dietary analysis requires a visit with a nutritionist. Physical impediments such as arthritis, back pain, and asthma should be identified and treated so as to optimize daily activity and adherence to exercise recommendations.