Disorders of Neutrophil Number
Neutrophilia
Neutrophilia, or granulocytosis, is usually defined as a neutrophil count greater than 10,000/mm3.
Etiology
Neutrophilia most often occurs secondary to inflammation, stress, or corticosteroid therapy. Cigarette smoking commonly causes neutrophilia as a result of inflammation in the airways and lungs. Malignancies, hemolytic anemia, and lithium therapy are less common causes. Neutrophilia is also associated with splenectomy. Extreme neutrophilia (i.e., neutrophil counts of more than 30,000 to 50,000/mm3), often called a leukemoid reaction, occurs with severe infections, sepsis, hemorrhagic shock, and severe tissue injury of any cause. Neutrophilia is also seen in patients with leukocyte adhesion deficiency (LAD), a rare disease in which neutrophils accumulate in the blood because they lack either the integrin CD11b18 or the selectin sLex (CD15s) required to leave the circulation.
Serious bacterial infections and chronic inflammation are usually associated with changes in both the number of circulating neutrophils and their morphology. Characteristic changes include increased numbers of young cells (bands), of cells with residual endoplasmic reticulum (Dohle bodies), and of cells with more prominent primary granules (toxic granulation). These changes are probably caused by the endogenous production of G-CSF or GM-CSF and are also seen with administration of these growth factors.
Primary neutrophilia (i.e., neutrophilia attributed to defects in proliferation and maturation of neutrophil precursors) occurs in patients with myeloproliferative disorders, such as chronic myeloid leukemia (CML) and polycythemia vera [see 5:VI The Polycythemias]. Hereditary and idiopathic neutrophilias have been described; they are benign and quite rare. One such uncommon idiopathic condition is Sweet syndrome, which is an acute febrile illness with painful cutaneous plaques and associated neutrophilia of any cause.19 Neutrophilia is also associated with congenital abnormalities. For example, infants with Down syndrome can have transient leukemoid reactions that must be distinguished from congenital leukemia.
Diagnosis
When neutrophilia cannot be readily attributed to an infection or inflammatory condition or to glucocorticosteroid therapy, the possibility of a myeloproliferative disease should be considered. The presence of splenomegaly, metamyelocytes, and myelocytes in the blood, together with increased basophils or eosinophils and a low leukocyte alkaline phosphatase (LAP) score, suggests CML [see 12:XVII Chronic Myelogenous Leukemia and Other Myeloproliferative Disorders]. A high LAP score or the presence of toxic granulations usually suggests an underlying infection. When there is uncertainty, bone marrow aspiration and biopsy, chromosomal analysis, as well as marrow cultures for bacteria (e.g., Salmonella, Brucella, Mycobacterium, and fungi), are warranted. The results of these tests will enable the clinician to make a diagnosis of CML (or another myeloproliferative disorder), a granulomatous infection, inflammatory disease, or metastatic malignancy. If no such cause can be found in an otherwise healthy-appearing person, a diagnosis of idiopathic or familial neutrophilia may be considered, and repeated neutrophil counts can be performed at monthly intervals until the diagnosis is clarified.
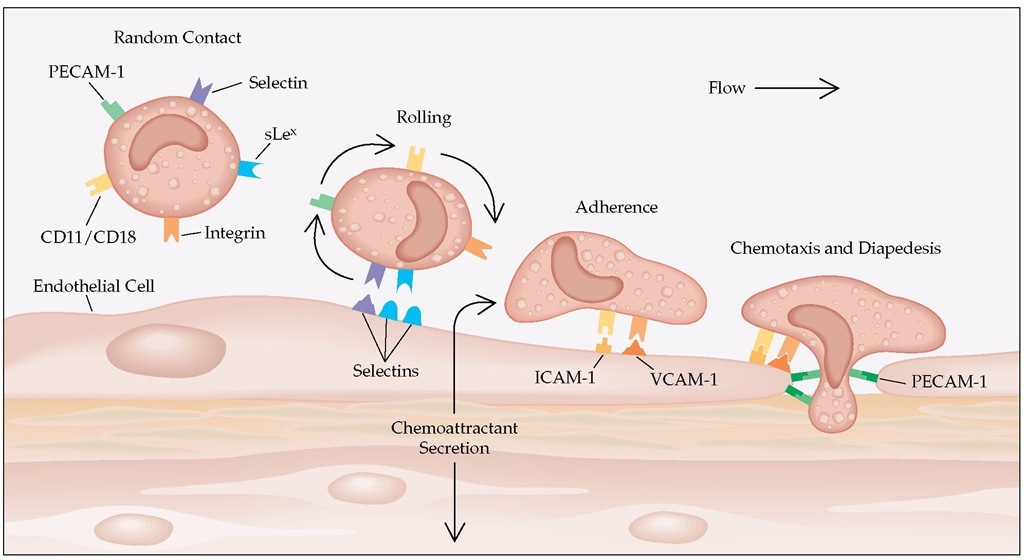
Figure 4 Neutrophils in the peripheral blood exist in either the circulating or the marginating pool. The marginated neutrophils roll along a vessel wall, where their surface carbohydrates interact with selectins on the endothelial cells. After activation by chemotactic agents, the neutrophils change shape and change the affinity of their integrin molecules for endothelial cell intercellular adhesion molecules. The neutrophils then crawl and undergo diapedesis by interacting with platelet-endothelial cell adhesion molecules on the endothelial surface and by liberating hydrolases that permit passage of the neutrophils through the capillary basement membrane. (PECAM-1—platelet-endothelial cell adhesion molecule-1; sLex— sialyl-Lewisx carbohydrate; ICAM-1—intercellular adhesion molecule-1; VCAM-1—vascular cell adhesion molecule-1)
Treatment
Except for the myeloproliferative syndromes, treatment of neutrophilia is not indicated. Neutrophil levels will return to normal when the inflammatory process is resolved.
Neutropenia
Neutropenia is generally defined as a neutrophil count of less than 1,800/mm3, which is two standard deviations below the normal mean. In some populations (e.g., Africans, African Americans, and Yemenite Jews), neutrophil counts as low as 1,000/mm3, or 1.0 x 109/L, are probably normal.
In otherwise healthy persons, the risk of bacterial infections is relatively low if the neutrophil count is greater than 500 mm3, or 0.5 x 109/L—the level usually defined as severe neutropenia. When neutropenia develops after myelotoxic chemotherapy, the risk of infection is much greater, particularly in patients whose age and medical history (e.g., diabetes, heart failure, renal failure, previous chemotherapy, and HIV infection) also predispose them to infection.22,23 Patients with neutropenia are also at greater risk for serious infections if they have disrupted mucosal or cutaneous barriers or are taking corticosteroids. Patients with neutropenia are at risk for infection by those pathogenic organisms that normally colonize body surfaces, particularly the skin, the orophar-ynx, and the GI tract. Thus, infections from staphylococci occur in neutropenic patients after breaks in the skin. Infection by mixtures of aerobic and anaerobic organisms of the oropharynx frequently causes gingivitis, pharyngitis, and sinusitis with neutropenia. Gram-negative bacilli often invade the blood from the GI tract in these patients. Antibiotic therapy, particularly therapy involving broad-spectrum antibiotics and protracted treatments, leads to colonization by resistant bacteria and to fungal infections.
Etiology
Neutropenia may be a primary or secondary condition. Primary neutropenia is caused by abnormalities of neutrophil formation derived from hematopoietic stem cells in the bone marrow; disorders with this underlying pathogenesis include the myeloid malignancies and several congenital disorders [see Primary Forms of Neutropenia, below]. Secondary neutropenia may be caused by drug therapy, infections, and immunologic disorders, including autoimmune diseases. Secondary neutropenia is far more common than primary neutropenia. In all of these conditions, the risk of infection depends on the level of blood neu-trophils and the capacity of the marrow to respond to an inflammatory stimulus and increase production of these cells. Usually if blood neutrophils are greater than 0.5 x 109/L, the risk of serious infection is relatively low.
Drug-induced neutropenia In aggregate, drug reactions are probably the most common cause of neutropenia in adults [see Table 2 ].25,26 Many cancer chemotherapy drugs, some of which are also used as cytotoxic immunosuppressive agents (e.g., cy-clophosphamide, methotrexate, and azathioprine), predictably cause dose-dependent neutropenia. The use of these agents requires careful attention to medical history, dosages, treatment schedules, and serial neutrophil counts to avoid serious and life-threatening toxicity. Other drugs cause neutropenia idiosyncratically. Many of these reactions probably occur because drugs can act as immunogens or as haptens, causing immunologic injury to neutrophils and their precursors. Other mechanisms of drug-induced neutropenia may involve direct toxicity of marrow cells in susceptible persons. Most patients recover from drug-induced neutropenia; the time for recovery can vary from 2 days to 2 weeks or more.
Infection-associated neutropenia Viral infections often cause mild neutropenia, especially in children. Such infections include measles and other viral exanthems, infectious mononu-cleosis, hepatitis, and HIV infection. The mechanisms are diverse. For example, in HIV infection, possible mechanisms include infections of the hematopoietic precursor cells and the marrow stromal cells, which lead to decreased production; induction of autoantibodies, which leads to accelerated turnover of mature neutrophils; and accelerated apoptosis of mature cells.27 HIV-associated neutropenia generally develops late in AIDS and is often compounded by the use of antiviral agents (e.g., zidovudine, ganciclovir), antibiotics (e.g., sulfonamides), or the presence of hematologic malignancies (e.g., lymphoma, Kaposi sarcoma).28 With other viral infections, the neutropenia is usually mild and without serious consequences. In rare instances, infectious mononucleosis causes severe hypoplasia, which has more severe consequences.29 Neutropenia and anemia are common features of human parvovirus B19 infection.
With severe bacterial infections, neutropenia occurs as a consequence of endotoxemia, which results in rapid neutrophil mobilization and turnover, especially in patients with a marrow reserve that is impaired because of previous chemotherapy, other drugs, or alcohol. In this setting, neutropenia generally portends a grave prognosis. Neutropenia occurs in parasitic infections associated with splenomegaly, such as kala-azar and acute malaria, presumably as a result of splenic trapping of the cells.
Autoimmune and idiopathic neutropenia Autoimmune neutropenia occurs as an isolated phenomenon or secondary to other autoimmune disorders.31 For example, in patients with Evans syndrome, autoimmune neutropenia may be associated with immune thrombocytopenia and hemolytic anemia. The bone marrow cellularity in patients with autoimmune neutrope-nia is either normal or increased, with a relative decrease in the number of cells in the late stages of the neutrophil formation. The diagnosis of autoimmune neutropenia requires specific anti-neutrophil antibody tests.32 The specificity of these tests probably varies considerably, and they are performed by a limited number of laboratories. It is often difficult to distinguish autoimmune neutropenia from cases otherwise categorized as idiopathic neu-tropenia. Neutropenia with antineutrophil antibodies also occurs in systemic lupus erythematosus,33 Sjogren syndrome,34,35 rheumatoid arthritis,36 and Felty syndrome (i.e., rheumatoid arthritis, splenomegaly, and neutropenia).
Patients with rheumatoid arthritis and neutropenia may have clonal expansion of large granular lymphocytes (usually CD2+, CD3+, CD8+, and CD57+ cells), which impair neutrophil production by excessive Fas ligand or interferon-gamma pro-duction.38 Recent studies indicate that this same mechanism may be involved in cases diagnosed as idiopathic neutropenia.39 The marrow typically shows increased lymphocytes with reduced neutrophils in the later stages of development. In most patients, the lymphocytosis is clonal and may evolve very gradually into a lymphoid malignancy.
Table 2 Drugs Associated with Neutropenia
|
ANALGESICS |
Phenytoin |
Methimazole |
PHENOTHIAzINES |
|
Aminopyrine |
Primidone |
Methylthiouracil |
Chlorpromazine |
|
Dipyrone |
Trimethadione |
Potassium perchlorate |
Methylpromazine |
|
ANTIBIOTICS |
ANTIHISTAMINES |
Propylthiouracil |
Prochlorperazine Promazine Thioridazine |
|
Cephalosporins |
Brompheniramine |
CARDIOVASCULAR AGENTS |
|
|
Chloramphenicol Clindamycin Doxycycline |
Cimetidine Ranitidine Thenalidine |
Captopril Diazoxide Hydralazine |
Trifluoperazine Trimeprazine |
|
Flucytosine |
Tripelennamine |
Methyldopa |
SEDATIVES AND |
|
Gentamicin |
Pindolol |
NEUROPHARMACOLOGIC |
|
|
ANTI-INFLAMMATORY AGENTS |
AGENTS |
||
|
Griseofulvin Tcnnia7in |
Fenoprofen |
Procainamide Propranolol |
Chlordiazepoxide |
|
lOWl liuZilU |
Gold salts |
Quinidine |
Clozapine |
|
Lincomycin Metronidazole |
Ibuprofen Indomethacin |
DIURETICS |
Desipramine Diazepam |
|
Nitrofurantoin Penicillins |
Phenylbutazone |
Acetazolamide Bumetanide |
Imipramine Meprobamate |
|
Rifampin |
ANTIMALARIALS |
Chlorothiazide |
Metoclopramide |
|
Streptomycin |
Amodiaquine |
Chlorthalidone |
|
|
Sulfonamides |
Dapsone |
Hydrochlorothiazide |
MISCELLANEOUS AGENTS Allopurinol Colchicine |
|
Vancomycin |
Hydroxychloroquine |
Methazolamide |
|
|
ANTICONVULSANTS |
Pyrimethamine |
Spironolactone |
Ethanol |
|
Carbamazepine |
Quinine |
HYPOGLYCEMIC AGENTS |
Levamisole |
|
Ethosuximide |
ANTITHYROID DRUGS |
Chlorpropamide |
Levodopa |
|
Mephenytoin |
Carbimazole |
Tolbutamide |
Penicillamine |
In sarcoidosis, cirrhosis, and congestive splenomegaly of diverse causes, neutropenia and thrombocytopenia often occur concomitantly, presumably because of splenic sequestration. In most instances, the neutropenia is mild and without recognizable consequences.
Other secondary causes of neutropenia Neonates can have severe neutropenia because of transplacental transfer of maternal IgG antibodies to the FcyRIII (CD16) isotype (previously called NA-1 or NA-2) that is inherited from the infant’s father.41 This abnormality is transient, usually lasting less than 3 months. Transient severe neutropenia also can occur in an infant as a result of transplacental transfer of other antibodies (e.g., transfer of IgG) from a mother with autoimmune neutropenia. Pure white cell aplasia is a rare acquired condition characterized by a complete absence of myeloid precursors.42 Pure white cell aplasia may be associated with a thymoma; if so, the aplasia may respond on removal of the thymoma. The short-term consequences of all these conditions depend primarily upon the level of blood neutrophils and the proliferative response of the marrow when inflammation or infections occur. The causes of other forms of neutropenia in children and adults are often difficult to establish and usually require referral to an expert hematologist.
Primary forms of neutropenia There are a number of congenital and inherited causes of neutropenia [see Table 3].
Diagnosis
Neutropenia is easily diagnosed by performing a white blood cell count and differential count. Patients with acute, severe neu-tropenia are often febrile and are frequently referred to as having acute febrile neutropenia. In this circumstance, attention is immediately focused on determining whether the patient has an infection, as well as focused on instituting empirical antibiotic therapy. Hematologic studies (e.g., bone marrow examination) are generally not necessary, because the cause of neutropenia is recognized from the patient’s history, and it will resolve if the inciting cause has been eliminated.
Initial evaluation of patients with chronic neutropenia should include a careful family history and review of the incidence and severity of infections, including oral ulcers, gingivitis, cellulitis, and more serious problems. A complete blood count will reveal whether the neutropenia is isolated or associated with other hematologic abnormalities. Medications should be discontinued if they can be implicated as causes of the neutropenia. A bone marrow biopsy and aspirate are indicated if there is any question of a primary disease affecting the marrow (e.g., metastatic carcinoma, tuberculosis) or if myelodysplasia or a hematologic malignancy is suspected. Serologic testing for infectious mononu-cleosis, hepatitis, and HIV is often warranted, as is measurement of antinuclear antibodies and rheumatoid factor titers. Broader immunologic assessments (i.e., lymphocyte subtypes and im-munoglobulin levels) are warranted if the history suggests a susceptibility to infections by viruses, parasites, or bacteria; and they are also useful to detect clonal proliferation of lymphocytes and to diagnose the large granular lymphocyte syndrome. Neu-trophil mobilization with corticosteroids and demargination tests with epinephrine are rarely helpful.
Treatment of Neutropenia
Evidence-based guidelines for management and prevention of acute febrile neutropenia associated with cancer chemotherapy have been developed by the Infectious Diseases Society of America (www.idsociety.org) and the American Society of Clinical Oncology (www.asco.org). Other guidelines are also available (www.guideline.gov) [see Table 4]. In general, acute management of severe, idiosyncratic, drug-induced neutropenia should be similarly managed.43,44
Table 3 Intrinsic Disorders of Neutrophils That Cause Neutropenia
|
Disorder |
Inheritance |
Clinical Features |
Diagnosis |
Treatment |
|
Congenital neutropenia (also known as infantile genetic agranulocyto-sis and Kostmann syndrome) |
AD, AR, S Locus: 19p13.3 |
From birth, upper respiratory, lung, liver, and skin infections; mild anemia; thrombocytosis; a normal immune system; possible development of leukemia |
Selective, severe neutropenia; marrow promyelocytes but few more mature cells; marrow eosinophils; normal chromosomes; possible G-CSF receptor defect Genetic testing: research only* |
G-CSF (effective in most cases); bone marrow transplantation; prophylactic antibiotics |
|
Myelokathexis |
AD, S |
Recurrent infections; severe leukopenia and neutropenia |
Marrow cellularity normal with maturing, often binucleate neutrophils |
G-CSF |
|
Cyclic neutropenia (also known as cyclic hematopoiesis) |
AD, S Locus: 19p13.3 |
Regular oscillations of blood cell counts, most prominently of neutrophil and monocyte counts |
Serial CBCs show severe neutropenia that recurs regularly, usually every 21 days Genetic testing: research only* |
G-CSF |
|
Shwachman-Diamond syndrome |
AR Locus: 7q11 |
Neutropenia with pancreatic insufficiency and sometimes with anemia or thrombocytopenia |
Neutropenia with malabsorption caused by pancreatic enzyme deficiency; tests for cystic fibrosis negative Clinical testing available* |
G-CSF; pancreatic enzymes |
|
Chediak-Higashi syndrome |
AR, S Locus: 1q42 |
Recurrent infections; partial albinism; lymphoproliferative syndrome; neutropenia; thrombocytopenia |
Giant cytoplasmic granules; defective neutrophil migration and bacterial killing Genetic testing: research only* |
Antibiotics; vitamin C; bone marrow transplantation |
|
Reticular dysgenesis and congenital immunodeficiency syndromes with neutropenia |
AR, S |
From birth, severe infections with severe leukopenia |
Neutropenia; hypogammaglobulinemia; T cell and B cell deficiencies |
Bone marrow transplantation; immunoglobu-lin therapy; G-CSF for neutropenia |
|
Dyskeratosis congenita |
AR Locus: Xq28; 3q21-q28 |
Severe infections; skin hyperpigmen-tation; dystrophic nails; leukoplakia |
Skin changes associated with severe neutropenia Clinical testing available* |
Prophylactic antibiotics |
AD—autosomal dominant AR—autosomal recessive CBC—complete blood count G-CSF—granulocyte colony-stimulating factor S—sporadic cases
Treatments for chronic neutropenia vary with the severity of neutropenia and the pattern of susceptibility to infection. Mild or moderate neutropenia (i.e., counts above 0.5 x 109/L, determined by serial counts over several weeks) rarely requires treatment. The neutropenia in Felty syndrome often responds to splenecto-my and weekly doses of methotrexate.45,46 With few other exceptions, long-term use of corticosteroids, ^-globulin injections, an-drogens, and splenectomy is not indicated for management of chronic neutropenia. With suspected infections, short-term, broad-spectrum antibiotic therapy is indicated, usually initiated after culture of blood and other body fluids for bacteria. Long-term antibiotic therapy is of unproven benefit in preventing infections, and it carries the risk of colonization by antibiotic-resistant organisms. G-CSF, usually in doses of 1 to 5 mg/kg/day, is of proven benefit for the treatment of congenital, idiopathic, and cyclic neutropenia and hastens the recovery of marrow from neu-tropenia after cancer chemotherapy.47 G-CSF and GM-CSF have been widely used to treat other forms of chronic neutropenia, including the neutropenia associated with HIV infection.