Definitions
The female reproductive system matures in a continuous, natural process from menarche to menopause, as the finite numbers of oocytes produced during fetal development are gradually lost to ovulation and senescence. Menopause is defined as the permanent cessation of menses1; by convention, the diagnosis of menopause is not made until the individual has had 12 months of amenorrhea. Menopause is thus characterized by the menstrual changes that reflect oocyte depletion and subsequent reduction in ovarian hormone production. However, the manifestations that occur around the time of menopause are caused by the underlying ovarian changes, rather than by the cessation of menstruation itself. Therefore, a woman who has undergone a hysterectomy but who retains her ovaries will experience normal menopausal symptoms as oocyte depletion leads to hypo-estrogenism, even though cessation of menstruation occurred with surgery.
Natural menopause occurs at or after 40 years of age and has no underlying pathologic cause [see Natural Menopause, below]. Induced menopause may occur after chemotherapy, pelvic radiation, or, most commonly, bilateral oophorectomy. Menopause is considered premature when it occurs before 40 years of age but is otherwise natural [see Premature Ovarian Failure, below].
The climacteric, a term now used infrequently, refers to the time of waning ovarian function associated with menstrual irregularity and vasomotor symptoms. Perimenopause is the time between the onset of the climacteric and the year after the last menses. Menopausal transition is replacing perimenopause and climacteric as the preferred term to describe the time of physiologic change around the cessation of ovarian function [see Figure 1].2 Premenopause is the entire reproductive span before onset of the menopausal transition, and postmenopause is the span of life after menopause.
In the past, natural menopause was considered to be an en-docrinopathy, with the ovary depicted as a failing organ and estrogen considered the optimal therapy. Given that menopause is a normal transition in the lives of most women and that significant risks have been associated with postmenopausal hormone "replacement," the viewpoint of menopause as an endocrinopa-thy is no longer espoused.
Natural Menopause
Epidemiology
The menopausal transition, which precedes menopause, has an average duration of 4 years, with a range of 0 to 10 years.3-5 The mean age at which menopause occurs in developed countries is 51 years4,6,7 and may be increasing.8 The standard deviation around this mean is about 2 years.4,9 Approximately 95% of women experience menopause by 55 years of age.4 Several factors appear to influence the age at which women experience menopausal symptoms and the final menstrual period; for example, menopause occurs approximately 1 year earlier in smokers6,7,10 and nulliparous women.6,7 Menopause may also occur earlier in women who have had ovarian cystectomies or unilateral oophorectomies.
Physiology and genetics of reproductive aging
Ovarian follicular depletion, by means of atresia, is the final common pathway in female reproductive aging. At 5 months of fetal age, the ovaries contain their peak number of primordial follicles, totaling approximately two million. At birth, girls have one million primordial follicles, approximately 25% of which remain at puberty. During the reproductive years, many follicles will begin to develop during each ovulatory cycle; all but one, the dominant follicle, become atretic. An estimated 1,000 follicles remain in the ovaries of a woman 51 years of age.12 Some poorly responsive follicles persist for a few years after the menopause.13 This progressive loss of follicles that accompanies aging is characteristic of all mammals studied to date; however, the controlling factors for this process have not been well defined.
Figure 1 The Stages of Reproductive Aging Workshop (STRAW) reproductive staging system showing the relationship of the final menstrual period with menstrual cycle changes and FSH serum concentrations.2 (FSH—follicle-stimulating hormone, T—elevated)
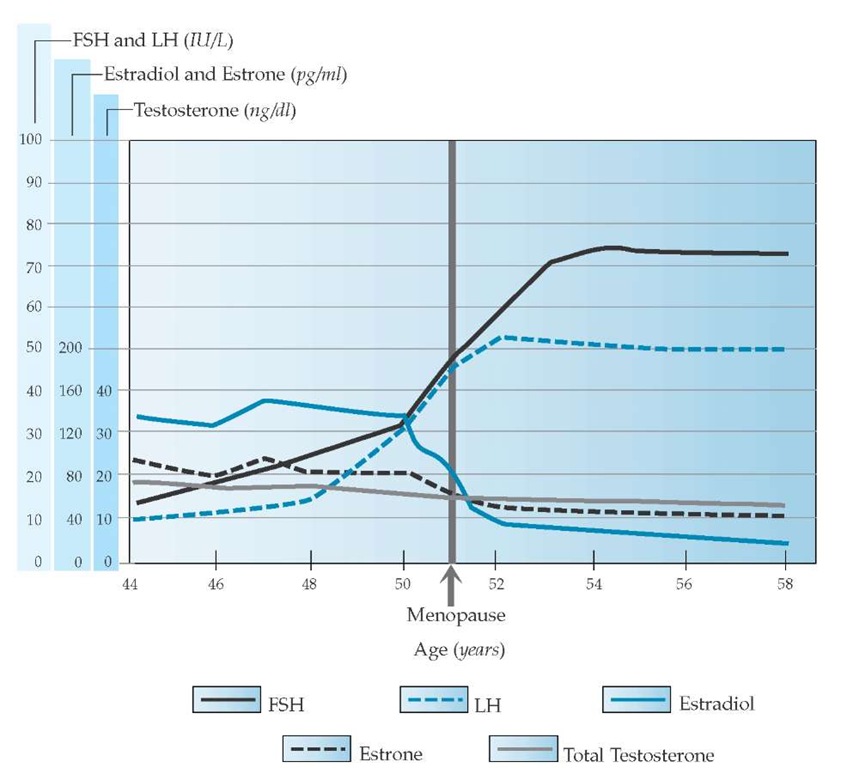
Figure 2 Approximate average serum concentrations of estradiol, estrone, FSH, LH, and total testosterone during the menopausal transition and postmenopause. A subtle rise in FSH occurs first, followed by a rise in LH and a decline in estradiol and estrone. There are no abrupt changes in testosterone, but a gradual continuous decline occurs that begins before the menopausal transition.
Beginning as early as 10 to 15 years before menopause, the length of the menstrual cycle progressively decreases, owing to a shortening of the follicular phase of the cycle. The observed decrease in cycle length continues until the onset of the meno-pausal transition, when both the average cycle length and the standard deviation of cycle length begin to increase as follicles are depleted and ovulation occurs less frequently.4,14 Insufficient follicular development results in inadequate estrogen production. With little estrogen available to stimulate the endome-trium, amenorrhea results.
There is good evidence that the timing of natural menopause is genetically programmed,15-17 but the specific genes involved are yet to be well defined. Common allelic variants of the estrogen receptor gene (estrogen receptor-a [ER-a] and ER-fi) contribute to the variability in the timing of menopause.18 In addition, all of the steroid receptors, as well as the proteins and enzymes involved in steroid biosynthesis and metabolism, are known to be coded by polymorphic sites (genetic changes found in at least 1% of the population). This genetic variability adds to the complexity of the actions and interactions of the reproductive steroids and to the timing and extent of menopausal symptoms.
Physiologic changes in menopause
Hormonal Changes
A subtle rise in the concentration of follicle-stimulating hormone (FSH) is the earliest and most consistent clinically measurable hormonal change noted in studies of reproductive aging.19,20 An FSH level measured during the early follicular stage of the menstrual cycle that is greater than two standard deviations above the mean level in women of reproductive age is a marker of impending menopausal transition.2 Luteinizing hormone (LH) levels remain normal initially, but they eventually become elevated as ovarian steroid secretion falls and gonadotropin-re-leasing hormone (GnRH) increases [see Figures 2 and 3]. The early selective increase in FSH appears to be caused by decreased secretion of the hormone inhibin B by the ovarian granulosa cells and is a marker of follicular atresia. Inhibin A and B, hormones that are involved in directing follicular development and were first characterized in the 1990s, suppress pituitary FSH produc-tion.21,22 As anovulation predominates, FSH and LH remain chronically elevated (i.e., there is a 10-fold to 20-fold increase in the FSH level and a threefold to fivefold increase in the LH lev-el),19,21,23and estradiol levels fall below 50 pg/ml [see Figure 2].
The physiologic changes that are associated with menopause are predominantly reflected by changes in circulating levels of estrogens, androgens, and progesterone [see Figures 2 and 3]. The hormonal system is made more complex by fluctuations in steroid hormones that alternate between free and bound states. Sex hormone-binding globulin affects serum levels of all steroid hormones, binding preferentially to testosterone, estrogen, and progesterone, in that order.
During the reproductive years, estradiol (E2) is the principal estrogen, both in quantity and in potency; estrone (E1) is present in a significant amount but is less potent than estradiol. Estriol (E3), a weak estrogen, is a metabolite of estrone and estradiol. Despite diminished fertility and ongoing follicular atresia, the ovulatory cycles of women in the menopausal transition have normal to high concentrations of circulating estradiol and es-trone. In fact, as women approach the menopausal transition, preovulatory estradiol levels can be higher than those seen in younger women.
After menopause, estradiol production drops by 90%,19,21 owing to follicular atresia [see Figures 2 and 3a]. What little estradiol is produced after menopause comes primarily from peripheral conversion of estrone. Estrone, the dominant estrogen after menopause, is produced through peripheral conversion of adrenal androstenedione by aromatase, primarily in adipose tissues [see Figure 3d]. Fatty breast tissue is a principal site of aro-matase activity, but activity is also present in the brain, muscle, liver, and, minimally, the ovary of a postmenopausal woman.
As reproductive aging progresses, serum levels of androgens decrease but not to the extent that estrogen levels diminish. An-drostenedione levels drop by approximately 50%,26-28 ovarian production declines [see Figure 3a], and adrenal output remains relatively constant [see Figure 3e]. Testosterone decreases by approximately 30% and continues to be secreted by the ovarian stroma, under the influence of LH [see Figure 3a].26-28 Serum concentrations of the adrenal androgen precursor dehydroepian-drosterone (DHEA) decrease with biologic aging, beginning before the final menstrual period [see Figure 3e].26
Figure 3 Multiple hormonal changes are associated with reproductive aging. (a) Within the ovary, secretion of inhibin B by granulosa cells decreases when a woman is in her mid-30s, and follicular depletion results in increasing rates of anovulation and diminished ovulatory surges of estradiol and estrone by her early 40s. Ovarian testosterone secretion continues; some ovarian testosterone is converted to estradiol by the enzyme aromatase, and the remainder is secreted as testosterone or the androgen precursor androstenedione. (b) In the menopausal transition, decreased circulating levels of inhibin and, subsequently, decreasing estradiol concentrations result in stimulation of the hypothalamus to increase secretion of GnRH. (c) Elevated circulating GnRH levels stimulate the anterior pituitary to increase secretion of FSH, followed by an increase in LH. Eventually, attempts by the brain to drive the ovary to produce estrogen fail, but production of androstenedione and testosterone by the ovarian theca cells continues in early menopause. (d) With diminished serum estrogen levels, adipocytes are stimulated to convert androstenedione to estrone via the enzyme aromatase. (e) Hormonal synthesis by the adrenal gland remains fairly constant, undergoing changes associated with aging, not menopause per se.
During the reproductive years, the principal source of progesterone is the corpus luteum; small concentrations of progesterone continue to be produced by the adrenal gland after the menopause [see Figure 3e].
The overall changes in reproductive steroid hormones observed following menopause include the following:
• Negligible estradiol production by the ovary
• A shift from the ovary to the adrenal gland as the primary source of estrogen precursors
• Emergence of estrone as the dominant estrogen
• Continued testosterone production by the ovarian stroma
• An overall increase in the ratio of androgens to estrogens
• A decrease in progesterone levels resulting from anovulation
Target Tissues
During the past decade, remarkable advances in the understanding of steroid biosynthesis, metabolism, and receptor tissue specificity have occurred.29 At least two estrogen receptors, ER-a and ER-fi, and two progesterone receptors, PRA and PRB, have been identified. Estrogen receptors are found in the genitourinary, cardiovascular, and gastrointestinal tracts and in the brain, bone, and integument. Different tissues have a predominance of specific receptors, depending on the individual’s endogenous hormonal profile. The complexity of the system leads to variations in the clinical manifestations of reproductive aging. Recent advances in the understanding of these complex physiologic processes have important implications for designing specific targeted therapies and have led to new classifications of pharmaceuticals: the selective estrogen receptor modulators (SERMs). Selective progesterone receptor modulators and selective androgen receptor modulators are also in development [see Preventive Health Care, below].
Endometrium During normal ovulatory cycles, progesterone, which is produced by the corpus luteum, causes the en-dometrium to mature to a secretory state. During the meno-pausal transition, endometrial shedding occurs less frequently because of anovulation, and oligomenorrhea results.4 Bleeding may be quite heavy in anovulatory cycles because estrogen is still produced, although at diminished levels, and stimulates the endometrium unopposed by progesterone. Furthermore, with increasing anovulation, longer cycles predominate and result in a thicker endometrium. Eventually, as anovulation predominates and estradiol production by the ovary becomes negligible, amenorrhea results.
Genitourinary epithelium The vagina is a principal target tissue for estrogen. Estrogen matures the vaginal epithelium, making it thicker and rugated. The estrogen-stimulated epithelial cells produce more glycogen, which in turn changes the bacterial flora and increases vaginal acidity.30 Hypoestrogenism results in thinning of the vaginal and vulvar epithelium. The base of the bladder is also derived from mullerian tissue and likewise is estrogen sensitive. Epithelial changes in the bladder are similar to those occurring in the vagina and vulva and result in thin, pale, friable tissues.
Central and sympathetic nervous systems Fluctuations in estrogen levels are associated with hot flushes.31 Hot flushes are caused by thermoregulatory dysfunction that is most likely initiated by the hypothalamus in response to estrogen withdrawal. Small elevations in core body temperature are followed by peripheral vasodilation. This results in a sensation of warmth and perspiration, both of which occur at a core body temperature that is lower than normal.32 To be susceptible to hot flushes, a woman needs to have been exposed to reproductive levels of estrogen and then experience estrogen withdrawal. For example, women with Turner syndrome, who never attain reproductive levels of estrogen, do not experience hot flushes.
Alterations in dopamine, norepinephrine, and serotonin pathways33-35 associated with systemic estrogen fluctuations may contribute to the vasomotor symptoms experienced during the menopausal transition and postmenopause. In addition to hot flushes and diaphoresis, symptoms may include a sense of prickling of the skin, heart palpitations, and anxiety.
Sleep disruption from vasomotor instability can result in in-somnia36 and daytime fatigue, and it may also contribute to mood and other neuropsychiatric changes.37 The prevalence of sleep-disordered breathing, including snoring and obstructive sleep apnea, increases after menopause,38,39 most likely because of the estrogen responsiveness of the upper airway muscula-ture.40 Other pathophysiologic alterations resulting in changes in cognition, mood, and sleep are not as well studied.
Women may experience a decline in libido during the meno-pausal transition or after the menopause.41 The contributing factors are complex and may include fatigue or stress (e.g., multiple responsibilities, including caretaking and employment), urogen-ital atrophy leading to dyspareunia, decreased testosterone levels [see Figure 2] (particularly in women who undergo surgical removal of both ovaries), sexual inactivity or dysfunction in a partner, and physical or emotional separation from a partner.
Bone Estrogen suppresses bone resorption. At the meno-pausal transition, bone resorption exceeds formation, and an accelerated loss in bone mass may occur.42 Bone mass may be lost at an annual rate of 3% to 5% in the first few years after the final menstrual period, but eventually, this rate of loss slows and continues at 1% to 2% a year.43 Trabecular bone, the predominant type of bone in the spine, hip, and distal radius, is affected first and to a greater degree than cortical bone [see 3:VI Diseases of Calcium Metabolism and Metabolic Bone Disease].
Cardiovascular system Cardiovascular risks44 and events45 increase after menopause. Among the factors that contribute to an increased risk of cardiovascular events are the levels of low-density lipoprotein (LDL) cholesterol and apolipoprotein B, which are higher after menopause.46 Estrogen receptors have been found in the muscularis of arteries in cardiovascular tis-sue.29 Estrogens appear to have a direct vasodilatory effect on the coronary artery, mediated by the formation and release of endothelium-derived relaxing factor, reduction of endothelin levels, and the promotion of prostacyclin production.
Coagulation factors Menopause has been associated with increases in factor VII, factor VIII, plasminogen activator in-hibitor-1 (PAI-1), and fibrinogen; all of these changes can lead to hypercoagulable states. Conversely, menopause has been associated with increased levels of antithrombin III and activated protein C, which may be beneficial in that these factors diminish coagulation.48 It is unknown whether these changes are caused by hypoestrogenism alone or by a combination of hormonal changes observed at the time of menopause.
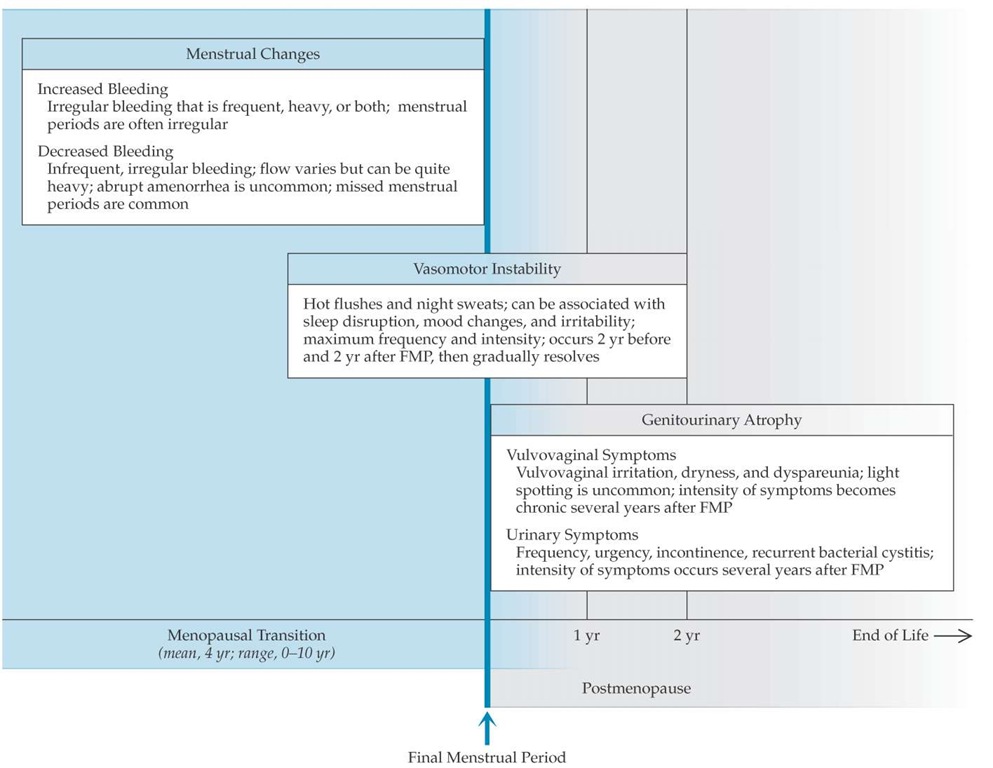
Figure 4 Characteristic symptoms of the menopausal transition and menopause. Peak vasomotor symptoms occur around the time of the final menstrual period. Menstrual changes are common before the menopause; abrupt amenorrhea preceded by normal cycles is unusual. Genitourinary atrophy is most common in postmenopause.
Integument A decrease in the production of dermal collagen and a subsequent reduction in dermal thickness49 result in significant changes in women’s skin, including wrinkles and dryness. In addition, a reduced rate of cutaneous wound healing has been associated with diminished secretion of transforming growth factor-p1 (TGF-p1) by dermal fibroblasts.50
Target-sensitive tissues and neoplastic growth Changes in the balance of reproductive hormones at the time of menopause have been associated with increased neoplastic growth in specific tissues. Hormonally sensitive neoplasms of the breast, colon, ovary, endometrium, and myometrium (leiomyoma) are widely recognized. Leiomyomas commonly increase in size during the menopausal transition but diminish in the postmenopausal period, presumably as a result of low levels of estradiol and progesterone. Other less common neoplasms occur in the gastrointestinal tract (esophageal and gastric)51; blood vessels; adipose and angiolymphatic tissues (angiomyolipoma,52 lymphangio-myomatosis)53; and the central nervous system (meningioma).54 All of these neoplastic tissues have been found to have reproductive hormone receptors and appear to be sensitive to steroid hormones. Contrary to previous evidence, recent observations suggest that melanoma is not progesterone sensitive.