Hyperlipidemia
A substantial number of patients with nephrotic syndrome without impaired renal function will have lipid abnormalities, including patients with diabetes and heavy proteinuria. The etiology appears to be related to an increase in hepatic synthesis of lipids. In an elegant study, Appel and colleagues demonstrated an inverse correlation between the total plasma cholesterol level and serum albumin level.48 There was also an inverse relationship between cholesterol level and plasma oncotic pressure. On the other hand, there was no correlation between cholesterol level and plasma viscosity. The incidence of hyperlipidemia is also increased in patients with CRF. Kasiske has estimated that approximately 30% of patients with CKD and proteinuria in the nonnephrotic range have total cholesterol values higher than 240 mg/dl. Levels of triglycerides and lipoprotein(a) are estimated to be higher than 200 mg/dl and 30 mg/dl, respectively, in approximately 60% of patients. High-density lipoprotein (HDL) cholesterol levels tend to be low, whereas only approximately 10% will have elevated low-density lipoprotein (LDL) values.49 One issue that must be considered is the relationship between lipid abnormalities and the deterioration of renal function. In a study by Bleyer and coworkers, there was no association between cholesterol level and a rise of 0.3 mg/dl in creatinine level, with measurements made at least 3 years apart.41 On the other hand, there are several studies, including studies with diabetic patients, that do demonstrate a relationship between lipid abnormalities and the progression of CRF. Because of the high incidence of cardiovascular disease, all patients with CRF should be screened. Recently, the guidelines for treatment were revised by the National Cholesterol Education Program.50 Diet should be the first line of therapy, but the addition of lipid-lowering drugs is almost always necessary. Newer HMG-CoA (3-hydroxy-3-methylglutaryl coenzyme A) reductase inhibitors lower both LDL and triglyceride levels. Fibric acid analogues are effective in reducing triglyceride levels; however, myositis and rhabdomy-olysis limit use of these agents. The dose of the fibric acid analogues should be adjusted appropriately for the degree of renal function.
Management of Complications of Chronic Kidney Disease
Sodium and water imbalance
As renal mass becomes progressively reduced, the fractional excretion of salt and water increases in the remaining nephrons. The solute diuresis that occurs in the remaining nephrons results in a relatively fixed level of salt and water excretion. Thus, in patients with CKD, renal salt and water excretion is limited to a range that is quite a bit narrower than that of healthy persons.
The optimal salt intake will differ from patient to patient; once a level has been prescribed, salt intake will need to be constantly monitored, because requirements will vary as renal function changes. The goal should be a salt intake that results in the patient’s being normotensive and maintaining a constant weight, with only trace edema present. A diet that restricts the amount of salt to 6 to 8 g/day is a useful starting point [see Table 5]. If the patient’s weight begins to decrease over a period of several days and the patient becomes more azotemic, a higher salt intake is required. In addition, during intercurrent illness,supplemental salt can be given in the form of bouillon cubes if a deficit in extracellular fluid volume develops. By contrast, if the patient’s weight increases over time and is accompanied by increasing edema and worsening hypertension, further salt restriction is indicated. Once the estimated GFR falls below 20 ml/min, even salt-restricted diets may exceed the excretory capacity of the kidney, and diuretic therapy will have to be utilized to prevent progressive expansion of the extracellular fluid volume.
|
Table 5 Typical Diet for Patients with Renal Insufficiency |
|
|
0.8 protein/kg body |
800-1,000 mg phosphate |
|
weight |
1,000-1,500 mg elemental |
|
6-8 g sodium |
calcium |
|
70 mEq potassium |
1,000-1,500 ml free water in excess of urine output |
The ability to maximally concentrate or dilute the urine becomes progressively impaired as renal function declines. As a result, patients with CKD are at risk of developing positive water balance and resultant hyponatremia, as well as negative water balance and hypernatremia. In general, fluid intake should be equal to urine output plus an additional 1,000 to 1,500 ml/day to account for insensible losses. The treatment of hyponatremia depends on the existing extracellular fluid balance. In volume-overloaded patients, further water restriction is indicated. In hy-povolemic patients, water restriction with judicious administration of salt and the withdrawing of diuretic therapy is the appropriate treatment.
Potassium imbalance
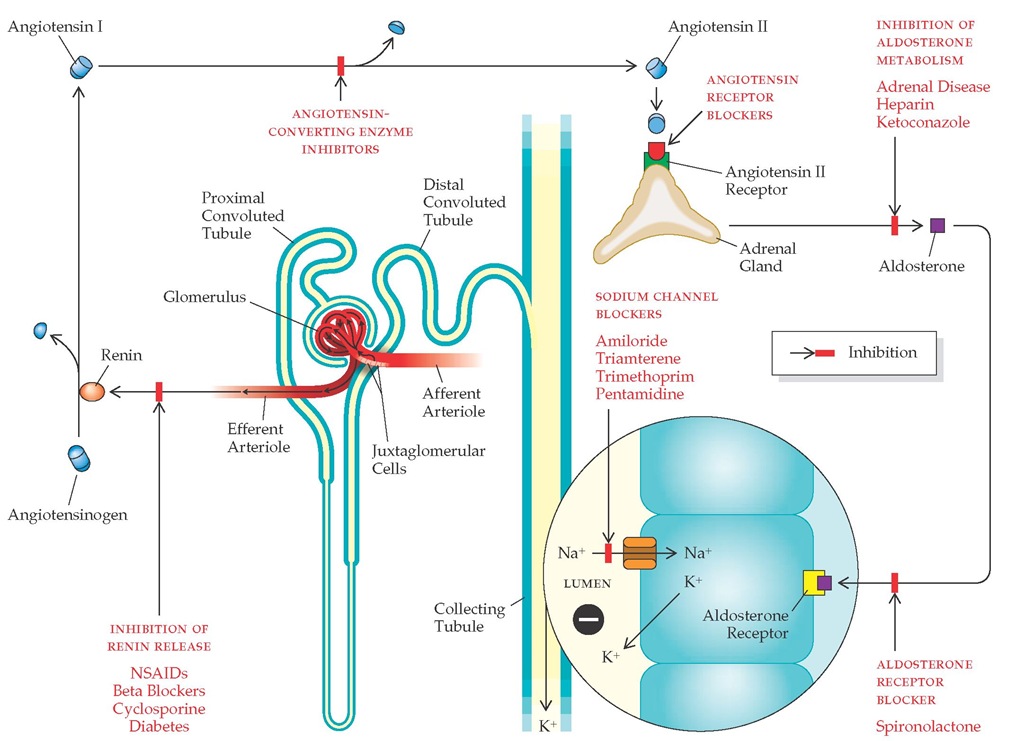
Potassium balance is generally maintained within normal limits until the GFR falls to less than 10 ml/min. This balance is achieved by an increased potassium excretion rate per remaining nephron and by an increase in extrarenal potassium excretion, primarily effected via the colon. The development of hyper-kalemia at higher levels of renal function suggests the presence of tubulointerstitial disease or disturbances in the renin-an-giotensin-aldosterone axis. In addition, there are several commonly used medications that can predispose to hyperkalemia in the patient with renal failure [see Figure 1].
The initial approach to the treatment of hyperkalemia is the institution of a low-potassium diet (50 to 70 mEq/day). Should hyperkalemia persist, administration of a loop diuretic is a reasonable second step, especially if the patient has demonstrable edema or is hypertensive. Loop diuretics increase distal sodium delivery and thus serve to increase potassium secretion from the distal tubule. If the patient is acidotic, sodium bicarbonate administration is an effective way to lower the serum potassium concentration. This agent also increases distal sodium delivery and, therefore, potassium secretion from the distal tubule. In addition, correction of the underlying acidotic state causes a shift of potassium into cells. Some patients continue to remain hyper-kalemic despite this therapy. In these patients, a potassium-binding resin such as sodium polystyrene sulfonate (Kayexalate) may have to be given daily or every other day. This agent should be given with a bowel cathartic such as sorbitol to prevent constipation. Constipation can actually worsen hyperkalemia because potassium secretion by the colon is substantial in patients with advanced renal insufficiency. Cathartics that contain magnesium should be avoided because of the risk of inducing hyper-magnesemia in the setting of renal insufficiency.
Figure 1 This diagram depicts the renin-angiotensin-aldosterone cascade. Aldosterone stimulates sodium reabsorption in the collecting duct, which in turn generates a lumen-negative potential. The luminal electronegativity serves as a driving force for potassium excretion. Drugs that interfere with this process are depicted according to mechanism of action. Use of these agents in the setting of chronic renal insufficiency can predispose to the development of hyperkalemia. (AI—angiotensin I; AII—angiotensin II; K—potassium; Na—sodium; NSAIDs—nonsteroidal anti-inflammatory drugs)
Metabolic acidosis
Under normal conditions, the kidney is responsible for regenerating consumed bicarbonate as a result of the buffering of daily net acid production. As renal insufficiency progresses, patients typically become acidotic. Initially, the acidosis is of the non-anion gap type, but as renal insufficiency becomes far advanced, an anion gap acidosis supervenes. If left untreated, aci-dosis leads to bone resorption; in addition, it may contribute to protein catabolism and can result in malaise and dyspnea.
Measurement and monitoring of the serum bicarbonate should be part of the routine electrolyte analysis in patients with CKD. In patients with stage 3 CKD, the bicarbonate should be measured at least every 12 months; in patients with stage 4 or 5 CKD, it should be measured every 3 months. Every effort should be made to keep the bicarbonate concentration above 22 mEq/L to avoid adverse effects on bone histology and protein catabolism.
Alkali therapy can be administered in the form of sodium bicarbonate tablets. Each 650 mg tablet contributes 8 mEq of bicarbonate. A useful starting dosage is one tablet three times a day. Alternatively, a sodium citrate solution (Bicitra) can be given; this solution contributes 1 mEq of bicarbonate per millimeter of solution. Citrate-containing alkali should not be administered to patients receiving aluminum-containing phosphate binders, because citrate is known to enhance the GI absorption of aluminum. Alkali therapy contains a substantial sodium load, and therefore, the patient needs to be monitored closely for the development of volume overload.
Calcium and phosphorus imbalance
Disturbances in calcium and phosphate metabolism regularly accompany CKD and contribute to many of the manifestations of uremia. As GFR declines, the serum phosphate level begins to increase, causing a reciprocal decrease in the serum calcium concentration. In response, parathyroid hormone (PTH) is released, resulting in increased phosphate excretion in each of the remaining nephrons; thus, calcium and phosphorus levels return to normal. As renal function continues to decline, calcium and phosphorus levels remain within the normal range but at the expense of an ever increasing level of PTH. Patients with stage 3 CKD commonly have elevated PTH levels and may already demonstrate evidence of osteitis fibrosa cystica on bone biopsy. Ultimately, loss of renal mass is so great that hyperphosphaturia per nephron is insufficient to prevent phosphate retention, so that hyperphosphatemia becomes sustained.
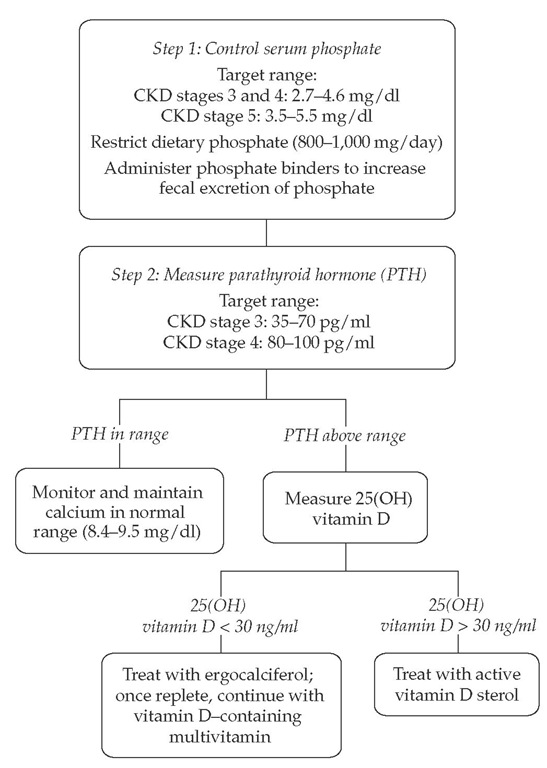
Figure 2 Steps to manage calcium and phosphorus abnormalities that accompany chronic kidney disease.
As renal mass declines, the circulating level of 1,25-dihydrox-yvitamin D [1,25-(OH2)D] also begins to fall. 25-Hydroxyvitamin D [25-(OH)D] undergoes 1a-hydroxylation in the kidney to form 1,25-(OH2)D. Lack of 1,25-(OH2)D contributes to the development of hypocalcemia because this hormone normally serves to increase calcium absorption from the GI tract and enhances the ability of PTH to mobilize calcium from bone. Decreased absorption of calcium from the intestine is further compounded by the low calcium content in the diet of patients with CKD. Low levels of active vitamin D also contribute to the development of secondary hyperparathyroidism, because this hormone normally exerts a direct inhibitory effect on the release of PTH from the parathyroid gland. Finally, during the course of advancing renal insufficiency, the set point at which calcium suppresses PTH release becomes higher, leading to further elevations in the level of PTH.
Clinical practice guidelines regarding the management of calcium and phosphorus disturbances in CKD patients are now available.52 The primary goal in the management of patients with CKD is to maintain the serum phosphorus level within normal limits [see Figure 2]. The serum level of phosphorus should be maintained between 2.7 and 4.6 mg/dl in patients with stage 3 or 4 CKD. In patients with stage 5 disease, the serum level of phosphorus should be maintained between 3.5 and 5.5 mg/dl. To achieve these levels, the patient should initially be placed on a phosphate-restricted diet (800 to 1,000 mg/day); the serum phosphorus level should be monitored monthly.52 Dietary sources particularly rich in phosphate must be restricted; these include eggs, dairy products (e.g., cream, milk, and cheese), and meat products. Although a few patients may be able to maintain the serum phosphate level within normal limits on a restricted diet alone, most patients with advanced CKD will require treatment with a phosphate binder to increase fecal excretion of phosphate.
Oral phosphate binders are available as either calcium- or non-calcium-containing drugs. In patients with stage 3 or 4 CKD, calcium-containing phosphate binders are usually effective in controlling the serum phosphorus. In patients with stage 5 disease, control of the serum phosphorus level may require a combination of both calcium- and non-calcium-containing binders.
The decision as to which class of binder to use should be based on the starting phosphate level and the calcium-times-phosphorus product. Every effort should be made to keep the calcium-phosphorus product lower than 55. In patients with a serum phosphorus level higher than 7 mg/dl or a calcium-phosphorus product greater than 63, a non-calcium-containing binder is the appropriate choice. Sustained use of calcium-containing binders in patients with a high product will result in the development of metastatic calcification; therefore, these binders should be avoided in such patients.
Sevelamer (RenaGel) is a calcium- and aluminum-free phosphate binder that is increasingly being used in the care of patients with ESRD. It has been shown to control serum phosphorus levels and to reduce PTH levels without inducing hypercalcemia.53 In addition, this agent lowers serum cholesterol levels. Several trials are comparing the degree of major artery calcifications associated with calcium-containing and calcium-free phosphate binders. Lanthanum carbonate is another non-calcium-containing binder that may soon become available for clinical use.
Because of concerns about long-term toxicity, aluminum-containing binders have largely fallen from favor. Aluminum-containing compounds such as aluminum hydroxide and aluminum carbonate may be used as a short-term therapy (1 month) but should be replaced thereafter by other non-calcium- containing phosphate binders, such as sevelamer. As soon as the serum phosphate level is reduced to less than 7 mg/dl and the calcium-phosphorus product is less than 63, calcium-containing binders can be utilized.
The calcium-containing phosphate binders are available as the calcium salts of carbonate, acetate, and citrate. Recent evidence suggests that calcium acetate is the most potent phosphate binder in this class. To be most effective, all the phosphate binders should be given with meals. The effectiveness can be further enhanced by varying the dose of the binder in proportion to the phosphate content of each meal.
As previously discussed, patients with advancing renal insufficiency tend to develop negative calcium balance because of decreased GI absorption of calcium and decreased calcium content in the diet. To remain in calcium balance, most patients with CKD require 1,000 to 1,500 mg/day of elemental calcium. This level is difficult to achieve with diet alone because many foods that are high in calcium are also high in phosphorus and are therefore restricted. To overcome this problem, supplemental calcium needs to be administered; supplemental calcium can be given as calcium carbonate or calcium acetate. When administered for this indication, calcium should be given between meals. Once again, to prevent calcium phosphate deposition in the tissues, calcium should not be given until the serum phosphate level is normalized.
Although calcium-containing binders provide an effective means of controlling phosphorus, their use may not be without risk. Calcium excess induced by the prescription of large doses of calcium-containing phosphate binders has been associated with calcifications of the aorta and the carotid and coronary ar-teries.54 Use of these drugs has also been implicated in the development of calciphylaxis. Given these concerns, the total dose of elemental calcium provided by calcium-based phosphate binders should not exceed 1,500 mg/day, and the total intake of elemental calcium, including that derived from dietary sources, should not exceed 2,000 mg/day.
Chronic kidney disease is associated with the development of secondary hyperparathyroidism. Monitoring of plasma levels of intact PTH may help prevent the development of secondary hy-perparathyroidism. In patients with stage 3 CKD, the target plasma level of intact PTH is 35 to 70 pg/ml; in patients with stage 4 disease, it is 80 to 100 pg/ml. For patients with values above the target range, 25-(OH)D levels should be obtained at first encounter; if the serum level is normal, the test should be repeated annually. If the serum level of 25-(OH)D is less than 30 ng/ml, supplementation with vitamin D2 (ergocalciferol) should be initiated. Once vitamin D levels are replenished, the patient should be maintained on a multivitamin containing vitamin D.
Serum levels of 25-(OH)D are considered the measure of body stores of vitamin D. In patients with a GFR of 20 to 60 ml/min, levels of 25-(OH)D below 30 ng/ml are common. The prevention and treatment of vitamin D deficiency in patients with stage 3 or 4 CKD is believed to decrease the frequency and severity of secondary hyperparathyroidism.
Serum levels of calcium and phosphorus need to be monitored every 3 months after starting therapy with ergocalciferol. If the total corrected serum calcium level exceeds 10.2 mg/dl, vitamin D therapy should be discontinued. If the serum phosphorus level exceeds 4.6 mg/dl, phosphate binders should be initiated or the dose increased. Persistently increased serum phosphorus levels should prompt the discontinuance of vitamin D.
In patients who have stage 3 or 4 CKD, serum 25-(OH)D levels greater than 30 ng/ml, and plasma levels of PTH above the target range, therapy with an active form of vitamin D (calcitriol, alfacalcidol, or doxercalciferol) is indicated. There were early concerns that administration of the active form of vitamin D would hasten the loss of renal function by causing hypercalcemia, hy-perphosphatemia, and hypercalciuria. Reports to date have generally shown no change in renal function in association with vitamin D therapy, provided that prolonged hypercalcemia is avoided. As a result, close monitoring of both the serum calcium level and the phosphate concentration are required, because vitamin D enhances the GI absorption of these electrolytes. Treatment should be initiated only if the total corrected serum calcium level is less than 9.5 mg/dl and the serum phosphorus level is less than 4.6 mg/dl. The serum levels of calcium and phosphorus should be monitored monthly for the first 3 months after initiation of therapy and every 3 months thereafter. The active vitamin D sterol should be held for calcium values that exceed 9.5 mg/dl or serum levels of phosphorus greater than 4.6 mg/dl.
Plasma levels of PTH also need to be monitored during therapy when the active form of vitamin D is being used. The target values for PTH in patients with CKD are higher than normal because of evidence that higher levels are required for normal bone remodeling, presumably as a result of the end-organ resistance to PTH in patients with uremia. Suppression of PTH to normal nonuremic values is not desirable, because such PTH levels are associated with a higher prevalence of a dynamic bone disease. After the initiation of therapy with vitamin D, plasma PTH levels should be measured every 3 months. Vitamin D should be withheld when PTH values fall below the target range.
Once patients reach stage 5 CKD, levels of PTH are almost always elevated. A plasma level of intact PTH of 300 pg/ml should prompt the initiation of active vitamin D therapy, with the goal of reducing PTH levels to a target range of 150 to 300 pg/dl. As with earlier stages of CKD, close monitoring of serum calcium and phosphorus levels is required. In these patients, treatment with ergocalciferol is not indicated, because there is inadequate renal mass to convert 25-(OH)D to the active vitamin D sterol.
Anemia
Patients with CKD almost uniformly develop a normocytic, normochromic anemia that tends to worsen in parallel with advancing azotemia. Anemia can develop in CKD patients who have a serum creatinine level as low as 2 mg/dl (occasionally lower), particularly in individuals with reduced muscle mass. The anemia seen in CKD patients is primarily caused by a decrease in the biosynthesis of erythropoietin from the kidney. Re-combinant human erythropoietin is now the most definitive treatment of the anemia of CKD. In addition to freeing the patient from repetitive exposures to blood-borne pathogens, iron overload, and sensitization, the use of erythropoietin has been demonstrated to improve cardiovascular and cognitive function and the overall quality of life of patients with CRF. Although transfusions are clearly indicated for the treatment of acute hemorrhage and cardiovascular instability, this form of therapy should no longer be considered routine in the management of anemia of patients undergoing peritoneal dialysis or hemodialysis.
Clinical practice guidelines are now available for the management of anemia in patients with CKD.1 Before the initiation of erythropoietin therapy, the patient should undergo a workup to exclude causes of anemia other than CKD. The evaluation should at least include hemoglobin levels, red blood cell indices, iron parameters, and testing for occult blood in the stool. Monitoring of iron levels should include testing for serum iron, total iron-binding capacity, percent transferrin saturation, and serum ferritin. If it is concluded that the anemia is the result of CKD, erythropoietin therapy can be initiated. Measurement of erythro-poietin levels is usually not indicated.
Erythropoietin is administered subcutaneously in doses of 50 to 150 U/kg up to three times a week. The target range for hemoglobin (hematocrit) is Hgb 11 to 12 g/dl (33% to 36%). The need to administer erythropoietin two to three times a week can be bothersome to the patient with early CKD who is largely asymptomatic and ambulatory. Darbepoetin alfa, an erythropoiesis-stimulating protein, offers the advantage of less frequent administration. Darbepoetin alfa has a much longer half-life than re-combinant human erythropoietin. Dosing once or twice weekly can often maintain hemoglobin concentrations in the target range. The optimum starting dose is 0.45 Mg/kg once or twice weekly administered subcutaneously.
Failure to respond to erythropoietin therapy is most commonly the result of iron deficiency. A transferrin saturation of less than 25% or a serum ferritin level of less than 100 mg/L indicates inadequate iron stores; such a condition requires iron supplementation, usually given as ferrous sulfate, 325 mg twice or three times a day. Oral iron is best absorbed when it is ingested without food or medications. Intravenous iron is usually reserved for patients who are already receiving hemodialysis or peritoneal dialysis. The occasional patient with early CKD who requires intravenous iron supplementation can be given 500 to 1,000 mg of iron dextran administered intravenously in a single infusion. An initial test dose of 25 mg should precede an infusion of 1,000 mg. During the course of therapy, the transferrin saturation and serum ferritin level should be monitored frequently to ensure that iron deficiency does not develop. Other causes of a suboptimal response include the presence of an underlying inflammatory illness, aluminum intoxication, and the presence of marrow fibrosis due to long-standing hyperparathyroidism.
Patients with advanced CKD typically develop a qualitative defect in platelet function. In patients at risk for bleeding complications, three forms of therapy have been shown to be effective in lowering the prolonged bleeding time associated with uremia. First, one can administer desmopressin intravenously at a dose of 0.3 mg/kg in 50 ml of normal saline solution infused over 30 minutes. Alternatively, one can administer cryoprecipitate (10 bags) infused intravenously over 30 minutes. Finally, conjugated estrogens given at a dosage of 0.6 mg/kg I.V. daily for 5 consecutive days have also been shown to be effective.
The Case for Early Referral to a Nephrologist
Optimal care of the patient with CKD involves a multifaceted treatment approach that includes close monitoring of renal function and aggressive institution of measures designed to slow the progression of loss of renal function. Interventions to reduce the comorbidities that accompany CKD should be initiated early. Metabolic and hematologic complications of uremia should be prevented; if already present, they should be treated judiciously. Patients with advanced CKD should be adequately prepared so that referral for renal replacement therapy is smooth and timely.
Disturbingly, recent reports indicate that pre-ESRD care in the United States is suboptimal for a substantially large number of patients.55-58 Evidence suggests that less than ideal management of the pre-ESRD patient may be an important factor contributing to the high morbidity and mortality of patients receiving dialysis. For example, many patients are significantly anemic and have not been treated with erythropoietin before initiation of dialysis. Severe anemia contributes to the development of left ventricular hypertrophy, which in turn is an important predictor of subsequent cardiac morbidity and mortality in patients receiving dialysis. Hypoalbuminemia is also a common finding at the time dialysis is initiated. Hypoalbuminemia is a strong predictor of subsequent morbidity and mortality in dialysis patients. Although the cause of hypoalbuminemia is multifactorial in this setting, it is likely that lack of supervision by a qualified dietitian early in the course of the disease is a contributing factor.
There is also evidence that many patients are not adequately prepared for initiation of dialysis. Optimal preparation for initiation of dialysis involves educating the patient and family about the various forms of renal replacement therapy. In those who choose hemodialysis, a vascular-access device needs to be placed several months before initiation so that it can be used for the first treatment. Because of the time commitment required to be trained for peritoneal dialysis, patients referred late are more likely to be started on hemodialysis, effectively limiting patient choice. The lack of a permanent access device at the time of initiation necessitates placement of a temporary catheter and increases the likelihood that the patient will receive an arteriovenous graft rather than an arteriovenous fistula. Finally, evidence is accumulating that early initiation of dialysis is associated with improved patient outcome.
Given the complexities involved in management, patients with CKD should be referred to a specialist for consultation and comanagement if the primary care provider cannot adequately evaluate and treat the patient. A nephrologist should participate in the care of patients who have an estimated GFR of less than 30 ml/min/1.73 m2. To achieve optimal management of all CKD patients, education targeting patients, generalists, and nephrolo-gists is required.