Malignant tumors can arise from cells of any layer of the skin— keratinocytes, melanocytes, fibroblasts, endothelial cells, or adipocytes—as well as from cells such as lymphocytes, which normally transit through the skin. Cutaneous metastases may also arise from other primary sites. In this topic, we review the most common malignant cutaneous tumors in their order of frequency.
Malignant Tumors of the Epidermis
Epidermal skin cancers are the most common cancers in humans. They arise in the keratinocytes and the melanocytes of the epidermis. Epidermal skin cancers present a unique opportunity for effective intervention with both early detection and primary prevention. They are amenable to clinical diagnosis by simple visual inspection and to pathologic diagnosis by minimally invasive biopsy.
Basal cell carcinoma (BCC) and squamous cell carcinoma (SCC) originate from the keratinocytes of the epidermis. Because these two cancers share many features, they are often lumped together under the term nonmelanoma skin cancer (NMSC).
Malignant melanoma is a malignancy arising from a melano-cyte. Although malignant melanomas can arise in any melano-cyte of the body, including the eye, the vast majority occur in the skin. Cutaneous malignant melanoma has been categorized into four major histogenetic types: lentigo maligna melanoma, superficial spreading melanoma, nodular melanoma, and acral lentiginous melanoma.
Sun exposure and skin cancer
Several lines of evidence implicate ultraviolet (UV) radiation in the pathogenesis of all three of the major epidermal skin can-cers.1 Epidemiologic data implicate long-term cumulative sun exposure in the development of SCC and intense intermittent sun exposure in the development of BCC and melanoma. Laboratory studies indicate that both UVA (320 nm to 400 nm) and UVB (290 nm to 320 nm) radiation from sunlight can damage DNA both directly and through oxidative damage. In addition, UV radiation can suppress the cutaneous immune system.2 The association of some SCCs with chemical carcinogens and the occurrence of acral lentiginous and mucosal melanomas in unex-posed areas of the body underscore the need for studies to identify additional etiologic agents.
Recognition of the important role of sunlight in the etiology of skin cancer affords an opportunity for primary prevention through the use of sun protection. Unfortunately, the exact timing and doses of UV exposure involved in the development of skin cancer in humans are not known and likely vary among the types of skin cancer. Accordingly, patients should be educated about the deleterious effects of sun exposure and tanning. Sun-protection efforts should be geared to an overall reduction of sun exposure through the avoidance of sun-seeking behavior and the use of sun-protective clothing. Broad-spectrum sunscreens with a sun protection factor (SPF) of 15 or greater are a useful adjunct to sun protection, but they should not be used to increase the amount of time spent in direct sunlight.3 The use of tanning beds should be avoided. The use of sunless tanning agents is safe, but the darkening of the skin that results from the use of these agents does not offer significant UV protection. For individuals who are assiduous in their sun protection efforts, attention should be given to adequate vitamin D intake through diet or supplements.
Nonmelanoma skin cancer
NMSC typically occurs as pink lesions on the sun-exposed skin surface. Any pink skin lesion that persists or recurs in the same location, especially if easily irritated by minor trauma, should raise the suspicion of NMSC. Some forms of NMSC will fade with changes in season (i.e., with reduced sun exposure) or with the application of topical steroids, and the clinician should advise patients that any lesion that recurs warrants further attention.
Basal Cell Carcinoma
BCC is a malignant cutaneous tumor arising from the basal keratinocytes of the epidermis.
Epidemiology BCC is the most common skin cancer. The reported incidence ranges from 3.4 per 100,000 per year in African Americans to over 1,100 per 100,000 per year in Townsville, Queensland, Australia.5,6 Although rare, metastases and death from BCC do occur.
Etiology and risk factors UV radiation—specifically, intense intermittent sun exposure—appears to play an important role in the development of BCC. Studies of basal cell nevus syndrome (Gorlin syndrome) have yielded dramatic insights into the genetics of BCC. The patched gene, which was first recognized as a developmental gene in the fruit fly Drosophila, has been identified as playing a critical role in the development of BCC. Almost all patients with basal cell nevus syndrome appear to inherit a mutated copy of the patched gene, and studies of sporadic BCC suggest that mutations in the patched gene pathway (i.e., the sonic hedgehog pathway) are a necessary and often sufficient step in the development of most BCCs.
Diagnosis The majority of BCCs occur on the head and neck. They occur in nodular and superficial forms, as well as in a variety of less common forms.
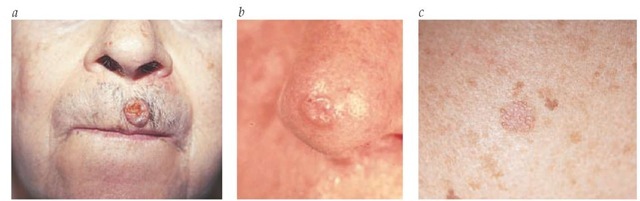
Nodular BCC appears as a raised, pearly, translucent, pink bump on the skin surface. It is often easily irritated, fragile, and associated with episodes of superficial ulceration or hemorrhage. When ulceration is prominent, it can lead to the appearance of a so-called rodent ulcer, in which the pearly translucent border is barely appreciable. Some nodular BCC lesions appear more white than pink and, on close observation, often demonstrate small telangiectasias. They tend to have a smoother, shinier surface and a firmer texture than common dermal nevi [see Figure 1].
Superficial BCC appears as a pink patch of skin. On close inspection, most superficial BCCs demonstrate a thready, translucent border, with areas of seemingly normal or slightly fibrotic skin within the lesion. Superficial BCC is usually found on the upper trunk, arms, and legs.
Figure 1 Nodular basal cell carcinoma—shown here above a patient’s lip, with a so-called rodent’s ulcer (a)—commonly presents as a raised, pearly, translucent pink bump on the skin surface (b). A superficial form appears as a pink patch of skin (c).
Less common clinical variants of BCC include morpheaform, pigmented, and cystic lesions. Morpheaform BCCs have an infiltrative pattern that histologically and clinically resembles a scar. Pigmented BCCs typically contain specks of blue-black pigment, but they may be deeply pigmented throughout. Pigmented lesions are most commonly a variant of nodular BCC. Cystic BCCs tend to be softer than typical nodular BCCs and may have a clear to blue-gray appearance.
Patient history plays a critical role in the diagnosis of BCC. When questioned about lesions that become easily irritated or bleed from minor trauma, patients can often alert the clinician to early lesions that would otherwise elude detection. With the patient under local anesthesia, a biopsy should be obtained of any suspicious lesion.
Differential diagnosis Nodular BCC can be confused with angiofibromas, dermal nevi, amelanotic melanoma, cutaneous metastases, dermatofibroma, and a host of benign adnexal tumors (e.g., trichoepithelioma). Superficial BCCs mimic several inflammatory dermatoses (e.g., eczema and tinea) and share several clinical features with actinic keratoses. Pigmented BCC can easily be confused with a primary melanocytic neoplasm. Cystic BCCs can be confused with cystic adnexal tumors and inflammatory lesions.
Treatment The goal of therapy is to adequately eradicate the lesion and ensure the best cosmetic and functional outcome. Multiple factors—such as the size, location, and histologic subtype of the lesions and attributes of the patient, including age, general health, skin color, and skin laxity—should be taken into consideration in choosing an optimal therapy.
The vast majority of BCCs are amenable to surgical treatment. The primary options include curettage and electrodesiccation, excision, and Mohs micrographic surgery. A small but significant subset of BCCs can be treated effectively with Mohs micro-graphic surgery, which entails microscopic examination of frozen sections of the entire undersurface of the excised specimen at the time of surgery. The technique may be indicated for recurrent lesions and lesions that have a high likelihood of recurrence. Such lesions include ill-defined lesions, large lesions (> 2 cm), lesions with a high-risk histology (i.e., aggressive growth pattern, sclerosing pattern, or perineural involvement), and lesions overlying embryonal fusion planes (e.g., ocular canthi or nasofacial sulcus). The cure rate of Mohs micrographic technique is significantly higher than the cure rates of other treatments of these high-risk lesions.8
Radiation therapy can be an effective, painless, and well-tolerated alternative that is typically reserved for older patients who are poor surgical candidates. Radiation therapy should be avoided, however, in patients with basal cell nevus syndrome. Cryotherapy is another therapeutic option for BCC in patients who are poor surgical candidates.
Topical therapy combined with pharmacotherapy using the immune response modifier imiquimod five times weekly for 6 weeks has been approved by the Food and Drug Administration for the treatment of superficial BCC of the trunk and extremities. One packet (250 mg) of imiquimod 5% cream is applied to 25 cm2 of affected skin.
Experimental therapies under investigation include intrale-sional chemotherapy, next-generation topical immune modulators, and photodynamic therapy.
All patients treated for BCC are at risk for local recurrence, and they are at significant risk for the development of additional skin cancers. Patients should be instructed in the self-examination of their skin, as well as in methods of sun protection. In addition, they should receive routine professional follow-up.
Prognosis The risk of local recurrence relates to the lesion’s size, location, and histology. Metastases are very rare: a prevalence of 0.0028% was reported in a series of 50,000 Australians.9 Metastases occur through both the lymphatic and the hematoge-nous routes; risk factors include basal cell nevus syndrome, im-munosuppression, and previous exposure to ionizing radiation. Metastases that are not amenable to surgical management are associated with a poor outcome.
Squamous Cell Carcinoma
Like BCC, cutaneous SCC arises from the keratinocytes of the epidermis. Histologically, the cells of well-differentiated SCC resemble the cells of the superior portion of the epidermis.
Epidemiology An estimated 150,000 to 250,000 new cases of cutaneous SCC were diagnosed in the United States in 1994.10 The estimated mortality from SCC in the United States in 1988 was approximately 0.5 per 100,000. Several lines of data suggest significant increases in SCC incidence. In Australia, for example,the incidence of SCC increased by 51% between the years 1985 and 1990.11 In the United States, some of the highest rates of NMSC have been detected in the southwest. A population-based survey in New Mexico found the incidence of SCC doubled in both males and females between 1978 and 1999.12
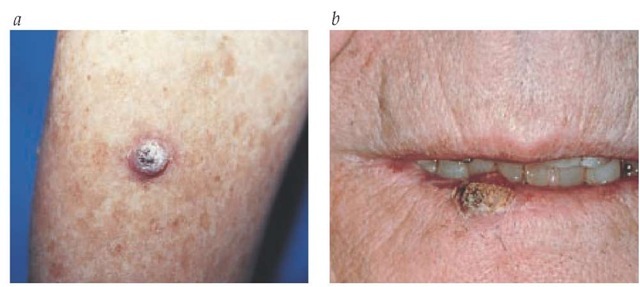
Figure 2 A squamous cell carcinoma is shown on an arm (a) and lower lip (b).
Etiology and risk factors In addition to sunlight, other known etiologic agents that contribute to the development of cutaneous SCC are ionizing radiation, chemical carcinogens, thermal burns, and chronic nonhealing wounds. Sun-related SCCs demonstrate a lower risk of metastases and death than SCCs related to other exposures. Factors involved in predisposition to SCC from sun exposure include light skin color, a tendency to burn, and an inability to tan.
Pathophysiology and pathogenesis Sun-related SCC is often associated with a precursor lesion called an actinic keratosis. Such lesions occur on the scalp, the face, the extensor surfaces of the forearms, and the backs of the hands. They tend to be rough-surfaced, irregularly shaped, and pink. They are often more readily felt than seen. The majority of patients with actinic ker-atoses have multiple lesions. The risk of SCC in these individuals has been estimated to be as high as 20%.13 SCC may also appear on normal-looking skin.
SCC of the oral or genital mucosa may arise in precursor lesions termed leukoplakia or erythroplakia. Mucosal SCCs are associated with a significant risk of metastases. Immune surveillance affects the progression of SCC. Immunosuppression, as occurs in transplant recipients and patients with lymphoma, is associated with a high incidence of SCC.14 In these patients, infection with human papillomavirus appears to play an etiologic role in conjunction with sun exposure. SCCs tend to be more aggressive in immunosuppressed persons.
Diagnosis Most lesions occur in areas of the body that are usually exposed to the sun. The lesions are pinkish, firm plaques that often have a rough, scaly surface [see Figure 2]. Biopsy is required for definitive diagnosis.
Differential diagnosis The differential diagnosis of SCC includes keratoacanthoma, Bowen disease, verrucous carcinoma, BCC, hypertrophic actinic keratosis, and common warts.
Keratoacanthomas share many features with SCC, both clinically and histologically. They arise de novo on normal-looking skin and grow very rapidly. They are typically pink, dome-shaped, shiny bumps with a central crateriform keratotic plug that occur on the surface of the skin. They may become very large. Although keratoacanthomas are not associated with a risk of metastasis, they can be locally destructive. Spontaneous regression of keratoacanthoma over the course of months has been well documented.
Bowen disease is SCC that is confined to the epidermis. It appears as red, scaly, minimally elevated plaques with well-defined, irregular borders. The reported association of Bowen disease with internal malignancy has not held up to closer scrutiny.
SCCs that lack a scaly keratotic surface can be confused with a host of other adnexal and dermal skin tumors.
Treatment Small SCCs evolving from an actinic keratosis can be adequately treated with simple curettage and electrodes-iccation. Larger actinic lesions, as well as lesions arising in non-sun-exposed areas of skin, are best treated with definitive surgical excision with confirmation of negative margins. High-risk, ill-defined lesions, especially those occurring in the surgically sensitive areas of the face, genitalia, hands, and feet, are often best treated by Mohs micrographic surgery.
Fractionated radiation therapy is an alternative treatment of primary SCC in older patients who are poor surgical candidates. The benefits of adjuvant radiation therapy are less clear, as are the benefits of sentinel lymph node biopsy and elective lymph node dissection (ELND) for patients with high-risk SCC of the head and neck.
Cytotoxic chemotherapy and biologic response modifiers have been used in patients who have advanced SCC; this therapeutic approach has been reported to have complete response rates of up to 68%, but there are few long-term survivors.16 Actinic keratoses are treated with cryotherapy, curettage, topical therapies (e.g., fluorouracil, imiquimod, or diclofenac), photody-namic therapy, and laser resurfacing to prevent progression to SCC.17 Regularly updated guidelines for the treatment of SCC and BCC are available through the National Comprehensive Cancer Network (NCCN).18
Prognosis Regardless of the therapy employed, high-risk lesions have a significant rate of local recurrence at 5 years. High-risk SCCs include those in specific anatomic sites (e.g., ears, lips, genitalia, and other non-sun-exposed areas), those greater than 2 cm in diameter, those with aggressive histologic features (depth > 4 mm, Clark level IV and above, and poorly differentiated histology), and those in immunosuppressed pa-tients.19 The primary route of SCC metastasis is via lymphatic spread to regional lymph nodes. Reported rates of metastasis vary from as low as 0.3% in small, sun-derived lesions to 33% in larger, poorly differentiated lesions.19 Reported overall 5-year survival rates for patients with regionally metastatic SCC have ranged from 25% to 47%.19
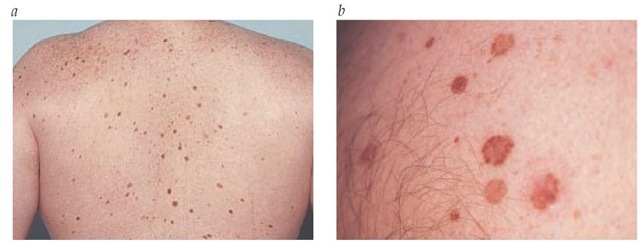
Figure 3 Dysplastic nevi typically are larger than common moles (a) and have variegate pigmentation and ill-defined borders (b).
Malignant melanoma Epidemiology
In the United States, a person’s lifetime risk for developing melanoma is about 1 in 75 (1.3%).20 Between 1973 and 1994, the incidence of melanoma rose by 121%, and the mortality rose by 39%.21 Encouraging trends include a shift toward the detection of earlier disease, as well as a stabilization of incidence rates in some segments of the population. In terms of both morbidity and mortality, however, the burden of melanoma-related disease continues to increase. Although melanoma can occur in anyone, it is primarily a disease of whites. Melanomas occurring in blacks are more commonly of the acral lentigi-nous variety.
Table 1 Adjusted Estimated Relative Risks of Melanoma by Nevus Type and Number25
|
Type |
Number |
Adjusted Relative Risk* |
|
0-24 |
1.0 |
|
|
Nevi > 2 mm and < 5 mm |
25-49 50-99 |
1.8 (1.3-2.5) 3.0 (2.1-4.4) |
|
> 100 |
3.4 (2.0-5.7) |
|
|
0 |
1.0 |
|
|
1 |
0.9 (0.7-1.3) |
|
|
Nondysplastic nevi > 5 mm |
2-4 |
1.3 (1.0-1.8) |
|
5-9 |
1.7 (1.0-2.7) |
|
|
> 10 |
2.3 (1.2-4.3) |
|
|
None |
1.0 |
|
|
Indeterminate |
1.0 (0.7-1.6) |
|
|
Dysplastic nevi |
1 2-4 |
2.3 (1.4-3.6) 7.3 (4.6-12.0) |
|
5-9 |
4.9 (2.5-9.8) |
|
|
> 10 |
12.0 (4.4-31.0) |
*Mutually adjusted and adjusted for age, sex, center, referral pattern, morphologic dysplastic nevi < 5 mm, sunburns, freckles, solar damage, scars, nevus excisions, and family history of melanoma (confidence interval = 95%).
Etiology and Risk Factors
Sun exposure Although strong epidemiologic and basic-science evidence supports an association between melanoma and sun exposure, the relationship appears to be complex.22 Lentigo maligna melanoma is associated with long-term cumulative sun exposure. Superficial spreading melanoma and nodular melanoma appear to be associated with intense intermittent sun exposure, especially in youth. Acral lentiginous melanoma has no apparent association with sun exposure. Basic-science studies and animal models have implicated different wavelengths of UV in melanoma carcinogenesis; UV wavelength may vary among types of melanoma.
Skin color Melanoma can occur in all racial/ethnic groups but is much more common in lighter-skinned individuals. Among whites, several additional risk factors have been identified, such as fair complexion, a tendency to burn, an inability to tan, freckling, and a family history of melanoma.22 Screening of the family members of patients with melanoma (particularly multiple melanomas) may be a useful preventive and diagnostic measure.23
Moles and dysplastic nevi The strongest phenotypic markers of melanoma risk are moles (nevi)—more specifically, increased numbers of moles and the presence of atypical moles (dysplastic nevi). Melanoma can arise in a preexisting mole or may arise de novo on normal-appearing skin.
Several epidemiologic studies have correlated dysplastic nevi with melanoma risk. Clinically, dysplastic nevi are large (> 5 mm) moles with variegate pigmentation and ill-defined borders [see Figure 3]. Histologically, dysplastic nevi are characterized by the presence of architectural atypia and random cytologic atypia. The degree of melanoma risk associated with dysplastic nevi depends on the genetic context. In families with familial melano-ma-dysplastic nevus syndrome, the abnormal mole phenotype appears to be inherited in an autosomal dominant fashion. Members of these families with dysplastic nevi have a lifetime melanoma risk that approaches 100%.24 Outside the context of familial melanoma, dysplastic nevi occur in approximately 5% to 15% of whites. In this general population, dysplastic nevi are markers of increased melanoma risk [see Table 1].