Treatment
The primary goals of therapy for Lyme disease are the control of inflammation and the eradication of the infection. Lyme disease is most responsive to antibiotics early in the course of the disease: erythema migrans typically resolves promptly and later-stage disease is prevented.58,59 Early localized infection that is limited to a single skin lesion, with mild or no systemic symptoms, is uniformly responsive to short-course oral therapy with a number of agents [see Table 1]. Of the antibiotics studied to date, the most effective agents for this stage of disease have been amoxicillin, 500 mg three times a day; doxycycline, 100 mg twice daily; and cefuroxime axetil, 500 mg twice a day. Each of these agents is taken for 14 to 21 days.60 An advantage of doxycycline is its efficacy against human granulocytic ehrlichiosis, a possible coinfection. Amoxicillin should be used in children and pregnant women. About 10% of patients with early-stage Lyme disease experience a Jarisch-Herxheimer reaction (higher fever, redder rash, or greater pain) during the first 24 hours of antibiotic therapy. Patients should be warned of the reaction; if it occurs, the symptoms may be treated with anti-inflammatory agents such as aspirin.
Table 1 Antibiotic Therapy for Lyme Disease
|
Disease Stage or Manifestation |
Agents and Dosage* |
Comments |
|
Early localized infection with a single skin lesion and mild or no systemic symptoms |
Amoxicillin, 500 mg p.o., t.i.d. x 14-21 days Doxycycline, 100 mg p.o., b.i.d. x 14-21 days Cefuroxime axetil, 500 mg p.o., b.i.d. x 14-21 days |
Doxycycline is also effective for human granulocytic ehrli-chiosis, a possible coinfection; amoxicillin should be used in children and pregnant women; Jarisch-Herxheimer reactions occur during the first 24 hr of antibiotic therapy in about 10% of patients |
|
Carditis with AV nodal block (PR interval > 0.3 sec) |
Ceftriaxone, 2 g/day x 14-21 days Penicillin G, 20 million units in four divided doses a day x 14-21 days |
Oral regimens are for first-degree AV block < 0.3 sec; for higher-degree AV block (> 0.3 sec), intravenous administration should be used for at least part of the treatment course |
|
Facial palsy with normal CSF findings |
Amoxicillin, 500 mg p.o., t.i.d. x 21-30 days Doxycycline, 100 mg p.o., b.i.d. x 21-30 days Cefuroxime axetil, 500 mg p.o., b.i.d. x 21-30 days |
Most experts prefer a 30-day course of treatment to reduce the likelihood of late neurologic relapses |
|
Meningitis and other neurologic disorders |
Ceftriaxone, 2 g/day x 14-28 days Penicillin G, 20 million units in four divided doses a day x 14-28 days |
Clearing of inflammatory CSF findings may lag behind bac-teriologic cure |
|
Arthritis |
Amoxicillin, 500 mg p.o., t.i.d. a 2 mo Doxycycline, 100 mg p.o., b.i.d. a 2 mo Cefuroxime axetil, 500 mg p.o., b.i.d. a 2 mo Ceftriaxone, 2 g/day a 1 mo Penicillin G, 20 million units in four divided doses a day a 1 mo |
Arthritis may persist despite appropriate antibiotic treatment; if PCR tests of joint fluid are negative, patients with persistent arthritis may be treated with anti-inflammatory agents or arthroscopic synovectomy |
*All treatment is with single agents.
AV—atrioventricular
CSF—cerebrospinal fluid
PCR—polymerase chain reaction
Although carditis resolves spontaneously, patients who have atrioventricular nodal block with a PR interval greater than 0.3 second should receive an intravenous regimen as at least part of the antibiotic course (e.g., ceftriaxone, 2 g/day, or penicillin G, 20 million units in four divided doses a day) for 14 to 21 days. Cardiac monitoring is recommended, but the insertion of a permanent pacemaker is not necessary.
Isolated facial palsies resolve completely or almost completely in nearly all patients. Patients who have facial palsy should undergo a careful neurologic evaluation, including a lumbar puncture and CSF examination. If facial palsy is the only clinical abnormality and CSF findings are normal, current practice is to administer oral antibiotics for 21 to 30 days. Most experts prefer a 30-day course of treatment, however, because of the late neurologic relapses that occasionally occur after shorter courses of therapy. Patients with evidence of active neuroborreliosis should receive a 2- to 4-week course of intravenous ceftriaxone or penicillin G.
Arthritis does not always respond to antibiotic therapy.62 About 10% of patients in the United States have persistent joint inflammation for months or even several years after 2 months or more of oral antibiotic therapy or 1 month or more of intravenous antibiotic therapy.62 Patients who have persistent arthritis despite appropriate treatment and negative PCR test results of joint fluid may be treated with anti-inflammatory agents or arthroscopic synovectomy.
B. burgdorferi has not been linked statistically to congenital anomalies, and no increased risk of an adverse outcome of pregnancy has been associated with asymptomatic seropositivity or history of previous Lyme disease.63 It is appropriate to maintain a lower threshold for institution of aggressive antibiotic therapy for suspected Lyme disease during pregnancy, but women should be reassured that no cases of fetal Lyme disease have occurred with currently recommended antibiotic regimens.
Despite receiving appropriate treatment for Lyme disease, a small percentage of patients continue to have subjective symp-toms—primarily, musculoskeletal pain, neurocognitive difficulties, or fatigue—that may last for years. This disabling syndrome, which is sometimes called chronic Lyme disease, is similar to chronic fatigue syndrome or fibromyalgia.64 In a large study, however, pain and fatigue were no more common in Lyme disease patients than in age-matched control subjects who had not had the disease.65 In a study of patients with post-Lyme disease syndrome who received either intravenous ceftriaxone for 30 days, followed by oral doxycycline for 60 days, or intravenous and oral placebo preparations for the same duration, there were no significant differences between the two study arms in the percentage of patients who said that their symptoms had improved, gotten worse, or stayed the same.66 There is no evidence that treatment of asymptomatic seropositive patients is beneficial.
Prevention
Primary prevention strategies will help reduce the number of Lyme disease cases, and some strategies may also preventother tick-borne illnesses, including babesiosis and human granulocyt-ic ehrlichiosis in the United States and tick-borne encephalitis in Europe. The first line of defense is avoidance of tick-infested habitats when possible; use of personal protective measures (e.g., repellents and protective clothing) in tick-infested habitats and checking for and removing attached ticks; and modifications of landscapes in or near residential areas. Tick control (burning or removing vegetation, acaricide use, and deer elimination) reduces I. scapularis populations by up to 94%, and acari-cide application to wildlife decreases nymphal 1. scapularis populations by up to 83%. Transmission of borrelial infection requires a period of 24 to 72 hours of tick attachment. Therefore, removal of ticks within 24 hours will usually prevent infection. If an engorged tick is found, a single 200 mg dose of doxycycline administered within 72 hours is effective at preventing the development of Lyme disease.67 A vaccine for Lyme disease (LYMErix), consisting of recombinant OspA and approved by the Food and Drug Administration for persons 15 to 70 years of age, was introduced in the United States in 1998 but was withdrawn from the market in 2002 because of low sales.
Leptospirosis
Leptospirosis is a worldwide zoonosis acquired by contact with infected animals or by exposure to contaminated soil or freshwater. A variety of animals can be chronically infected and shed viable organisms that can persist in the environment, leading to human infection. The importance of environmental and occupational exposure is reflected by several of the synonyms for this disease: rice-field fever, cane-cutter fever, swamp fever, and mud fever. Clinically, leptospirosis can range from asymptomatic infection to severe multisystem disease with significant mortality. Leptospirosis cases are often undiagnosed because of the protean manifestations of infection, lack of awareness by clinicians, and limited diagnostic tools.
Microbiology
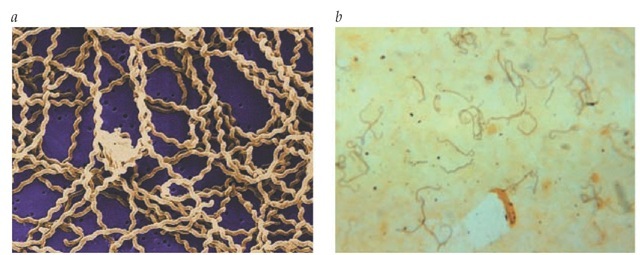
The genus Leptospira consists of tightly coiled spirochetes that are thin (0.1 ^.m) and vary in length from 6 to 20 ^.m [see Figure 4]. Leptospira can be cultured on artificial media, but initial cultures may take weeks to grow.69
In the past, the genus Leptospira was divided into two species, the pathogenic L. interrogans and the nonpathogenic L. biflexa, on the basis of serologic reactivity to the lipopolysaccharide O antigen. A large number of serovars have been identified within each of these species. The classification of the genus Leptospira has undergone extensive reorganization with the application of DNA-relatedness techniques. Thirteen named species and four unnamed genomospecies are now recognized.70 The complete genetic sequence of L. interrogans serovar lai has been determined.71
Epidemiology
A wide variety of mammals can become infected with Lep-tospira and serve as reservoir hosts. Particularly important in human infection are rodents, livestock, and pets.72,73 Animals are often infected early in life and develop persistent infection of the proximal renal tubules. Infected animals are frequently asymptomatic and shed viable spirochetes in the urine for extended periods, contaminating water and soil. Leptospira can persist in a warm, moist environment for several weeks—a fact that correlates with the higher incidence of human infection in tropical areas, particularly during the rainy season. In temperate regions, where survival of the organism in the environment is limited by temperature, the incidence of leptospirosis is seasonal, peaking in the summer and early fall. In the United States, the greatest number of cases have been reported in Hawaii, but cases occur throughout the country. Studies have demonstrated lepto-spiruria in 41 of 500 healthy dogs in Kansas and 35% of Texas cattle.
Figure 4 (a) A scanning electron micrograph reveals numerous corkscrew-shaped Leptospira spirochetes atop a 0.1 pm polycarbonate filter. (b) Leptospira spirochetes are visible on a photomicrograph of a liver smear, using a silver-staining technique, taken from a patient with a fatal case of leptospirosis.
Human infection can result from direct contact with urine or tissues of infected animals; such exposure occurs in veterinarians, dairy workers, hunters, and animal handlers. The more common exposure is to contaminated water and soil.76 Outbreaks of leptospirosis have been reported related to ecotourism and adventure sporting events.77,78
Pathogenesis
Leptospires enter the body through minor cuts and abrasions, mucous membranes, and conjunctiva and by inhalation of infected aerosols. Infection spreads throughout the body via the bloodstream. Motility and the ability to migrate through tissues are felt to be important to the pathogenesis of Leptospira, allowing the spirochete to establish initial infection and to disseminate to sites of end-organ damage.71 A variety of hemolysins and phospholipases and a sphingomyelinase have been identified and may have a role in the ability of this organism to move through tissues. The mechanism by which Leptospira causes tissue damage is not fully understood, but a systemic vasculitis may facilitate migration of the organism into a variety of tissues, accounting for the broad spectrum of clinical illness.79 In addition, the host immune response is felt to contribute to tissue damage and the clinical severity of disease.80 In animals with experimental infections that mimic the more severe icteric Weil disease and hemorrhagic syndromes, the livers and kidneys demonstrate large numbers of leptospires and associated tissue inflammation. Several leptospiral proteins have been identified with homology to host proteins important in hemostasis, which may activate hemolytic pathways and contribute to the hemor-rhagic complications seen in severe disease.81
Diagnosis
Clinical Manifestations
Infection with Leptospira can result in a spectrum of clinical manifestations. Asymptomatic infection is not uncommon, and subclinical or very mild disease that does not lead to medical attention has been reported in several studies.82,83 When symptomatic, leptospirosis has classically been described as a biphasic illness, with a self-limited septicemic phase followed (although not invariably) by an immune phase. Symptoms of infection develop after an incubation period of 2 to 20 days (mean, 10 days). Illness begins abruptly with high fevers, chills, rigors, headache, myalgias, abdominal pain, nausea, vomiting, diarrhea, and cough. Conjunctival suffusion and muscle tenderness, particularly of the calf and lumbar areas, have been cited as distinctive examination findings. Less common physical findings include lymphadenopathy, splenomegaly, and hepatomegaly.84 The acute illness lasts 5 to 7 days. Leptospira can be recovered from the blood and cerebrospinal fluid during the first week of illness, although symptoms of meningitis are not prominent.
The septicemic phase may be followed by a period of improvement in symptoms and absence of fever lasting several days. The onset of the immune phase of illness coincides with the development of specific antibody to Leptospira and the clearance of organisms from the blood and CSF. Leptospires remain detectable in the kidney, urine (leptospiruria), and aqueous humor for several weeks. The immune phase of leptospirosis is often more severe than the septicemic phase and is potentially fatal. The immune phase lasts 4 to 30 days and is characterized by aseptic meningitis, uveitis, iritis, rash, hemorrhagic pneumonitis, and hepatic and renal involvement.71,84-88 Mortality in patients with severe disease results from multiorgan failure and pulmonary hemorrhage.
The septicemic and immune phases of leptospirosis are illustrated by the meningeal findings. Early in infection, patients often have severe retro-orbital headaches and photophobia. CSF studies reveal a neutrophilic pleocytosis, with counts ranging from 10 to 1,000 cells/^l. Leptospira can often be demonstrated in the CSF by culture and PCR. During the immune phase of illness, approximately 25% of patients will develop an aseptic meningitis characterized by headache, vomiting, and signs of meningeal irritation. CSF analysis reveals a lymphocytic pleocy-tosis, and Leptospira can no longer be isolated.
Acute renal failure is reported in 16% to 40% of cases and is associated with tubular necrosis and interstitial nephritis. Jaundice develops in approximately 40% of patients and does not seem to result from hepatocellular damage but, rather, from cholestasis.84 Serum bilirubin levels may be very elevated (usually to less than 20 mg/dl), with more moderate elevations in transaminase concentrations (less than 200 IU/L) and mild elevation of the alkaline phosphatase level.89 Bilirubin levels may take days to weeks to normalize. Pulmonary involvement occurs in up to 70% of cases, and symptoms may range from cough, dyspnea, and hemoptysis to respiratory failure. Severe hemorrhagic pneumonitis and acute pulmonary distress syndrome may occur in the absence of hepatic and renal failure.90 Radiographs reveal patchy alveolar infiltrates that may progress to large areas of consolidation that are thought to represent pulmonary hemorrhage. Significant pulmonary involvement is a poor prognostic sign and is associated with increased mortality.84
Weil disease is the most severe form of Leptospira infection, with a mortality of up to 40%. It may develop during the immune phase of a biphasic illness or progress directly from the acute phase without the characteristic brief improvement in symptoms to fulminant illness. Weil disease is characterized by high fevers and the rapid onset of liver failure, acute renal failure, hemorrhagic pneumonitis, cardiac arrhythmias, and circulatory collapse.71,91
General Laboratory Studies
During the initial septicemic phase of illness, results of routine laboratory tests are not specific; the neutrophil count may be normal or elevated. During the immune phase of illness, the laboratory values are consistent with the specific end-organ dysfunction. Liver dysfunction is often characterized by markedly elevated serum bilirubin levels with less pronounced increases in serum transaminase and alkaline phosphatase levels. Renal function can deteriorate rapidly, with evidence of interstitial nephritis on biopsy. Renal injury may be compounded by associated hypovolemia. In patients with evidence of meningitis, CSF assays reveal a neutrophilic pleocytosis early and, later, a lymphocytic pleocytosis (usually below 500 cells/^.l); modest elevation in protein levels (50 to 100 mg/ml); and a normal glucose level.
Specific Laboratory Studies
Leptospirosis can be diagnosed in three ways: by the direct demonstration of organisms in blood, urine, or tissues; by culture; or serologically.
Microscopy Dark-field microscopy of blood or urine and silver staining of infected tissues have been used to directly visualize leptospires, but both techniques have limited sensitivity and specificity.92 Approximately 104 leptospires/ml are necessary for one cell/field to be visible by dark-field microscopy.93 Immunostaining has been shown to enhance the ability to directly demonstrate leptospires in tissue; however, the reagents are not commercially available.94,95
Culture Leptospira grows slowly in the laboratory, which can limit the utility of culture for diagnosis. Leptospira can be isolated from a variety of body fluids using commercially available semisolid, albumin-polysorbate media. Antibiotic use may limit the sensitivity of culture, and the timing of specimen collection is important. During the first 7 to 10 days of illness, the spirochete can be recovered from blood, particularly during periods of fever, and from CSF. The culture media should be inoculated with several drops of the patient’s blood or CSF at the bedside. Alternatively, blood specimens can be collected in heparin or sodium oxalate (citrate anticoagulation is inhibitory); these should be inoculated within 24 hours. Recovery of leptospires from the urine begins after the first week of illness, and as with blood and CSF, inoculation of media should be done promptly (in less than 1 hour after collection). The cultures are incubated at 30° C and are not reported as negative until after a minimum of 6 to 8 weeks. Identification at the species level is available from a few reference laboratories.
Serology The mainstay for the diagnosis of leptospirosis has been by serology, using a microscopic agglutination test (MAT).96 The end point is the highest dilution of serum in which 50% agglutination occurs. The MAT uses live organisms, which are coincubated with the patient’s serum and examined by dark-field microscopy for agglutination. A fourfold rise in paired titers or a single titer of greater than 1:800 in a patient with an appropriate history supports the diagnosis. Many patients will have a negative test result during the acute illness, and serocon-version may be delayed as long as 30 days after the onset of clinical illness. The MAT is technically complex to perform and to interpret. In addition, live cultures must be maintained for all the serovars required as antigens.
Because of the complexity of the MAT, rapid screening tests for leptospiral antibody have been developed. IgM antibody becomes detectable during the first week of illness and is more sensitive than MAT when the first specimen is taken early in the illness. Commercially available IgM dipstick assays have been shown to perform well.
DNA amplification An attractive method to confirm a diagnosis of leptospirosis is PCR, which is more sensitive than culture and is particularly useful in early infection, before significant antibody titers develop. Leptospira DNA has been amplified from blood, urine, and various tissues.94,99 However, the PCR test is currently limited to research laboratories.
Treatment
Antibiotic therapy does not appear to have a clear advantage over placebo for treatment of leptospirosis but does tend to reduce mortality, duration of fever, length of hospitalization, and extent of leptospiruria.100 Most practitioners treat patients with severe disease with parenteral penicillin (1.5 million units every 6 hours), ampicillin (1 g every 6 hours), or ceftriaxone (1 g daily), each for 7 days. Mild infections can be treated with oral doxycy-cline (100 mg every 12 hours), ampicillin, or amoxicillin. Jarisch-Herxheimer reactions have been reported after treatment with penicillin and ampicillin.101,102
Prevention
Prevention of leptospirosis can be achieved by limiting high-risk exposure with appropriate protective measures. These include not swimming in potentially contaminated freshwater, wearing rubber gloves and goggles when handling animals, and not walking barefoot. Weekly doxycycline prophylaxis has been shown to be effective at preventing symptomatic infection in persons at high risk for exposure.103
Relapsing Fever
Relapsing fever, caused by spirochetes of the Borrelia genus, is a febrile illness characterized by recurrent episodes of fever and septicemia separated by afebrile periods. Two forms of the disease are recognized: louse-borne and tick-borne.
Figure 5 Ornithodorus, or soft tick, is the vector of the Borrelia species that causes tick-borne relapsing fever. Adult ticks are approximately 2.5 mm in size—about the size of a sesame seed.
Epidemiology
Louse-borne relapsing fever (LBRF) is caused by infection with B. recurrentis. This spirochete is transmitted from person to person by the human body louse (Pediculus humanus), and humans are the only known reservoir for this organism.104 LBRF usually occurs in epidemics that are associated with social catastrophes, such as war and famine, which foster the conditions (e.g., crowding and poor hygiene) that allow lice to flourish and spread from person to person. In addition, LBRF is endemic in areas of central and eastern Africa and Peru.105 Humans become infected by crushing the lice, which releases the spirochetes and permits them to penetrate the skin or mucous membranes.
Tick-borne relapsing fever (TBRF) is caused by at least 15 different species of Borrelia, each of which is associated with a distinct member of the soft ticks of the genus Ornithodoros. In contrast to Dermacentor and 1xodes ticks, which are teardrop shaped with mouth parts visible dorsally, Ornithodoros ticks have an oval body shape and mouth parts located on the ventral surface (not visible dorsally) [see Figure 5]. Adult Ornithodoros ticks are about the size of a sesame seed. Animal reservoirs for these Borrelia species include rodents and small animals, such as squirrels, rabbits, chipmunks, owls, and lizards. Ornithodoros ticks are obligate blood feeders that typically inhabit caves, decaying wood, rodent burrows, and animal shelters. Their bite often goes undetected because they feed rapidly (over 5 to 20 minutes) at night and have a painless bite.106 Infection of humans occurs when the tick releases saliva or excrement during feeding. TBRF has been reported worldwide, with the exception of Antarctica, Australia, and certain areas in the southwestern Pacific. Most cases in the United States occur west of the Mississippi River, particularly in the mountain and high-desert areas.107 Human infection occurs with activity that brings the person into the tick’s environment (e.g., camping and cave exploration).
Pathogenesis
After entering the bloodstream, Borrelia spirochetes multiply and can reach levels of 105 to 108 organisms/^l. These periods of spirochetemia are associated with fever and systemic symptoms. With the development of a specific antibody response, the spiro-chetes are cleared from the blood, becoming sequestered in internal organs, and patients become asymptomatic. In response to immune pressure, the Borrelia organisms undergo modifications of their outer-surface proteins that allow the spirochetes to reemerge into the circulation; reemergence results in recurrence of symptoms.108 This process of antigenic variation in response to specific antibody formation can occur for a number of cycles and is responsible for the relapsing course of clinical disease. The number of febrile relapses is higher for tick-borne infection than for louse-borne infection.
Clinical manifestations
The incubation period of relapsing fever can range from 4 to 18 days and averages about 1 week. In both TBRF and LBRF, illness begins with the abrupt onset of fevers (usually above 39° C [102.2° F]), rigors, myalgias, arthralgias, and severe headache.107,109 Physical findings include conjunctival suffusion, petechiae, abdominal tenderness with hepatosplenomegaly, and altered sen-sorium. A truncal rash that can be petechial, macular, or papular is common during the primary febrile episode. Almost a third of patients develop neurologic complications, including meningitis, cranial nerve palsies, coma, and seizures.
The primary febrile episode is unremitting until it terminates abruptly after 3 to 6 days. After a period of well-being lasting 7 to 10 days, symptoms recur. Each relapse is usually shorter in duration and less severe in intensity. LBRF often is associated with a single relapse, whereas TBRF often is associated with multiple symptomatic relapses.
Mortality from relapsing fever ranges from 2% to 40% and is higher with LBRF. Death is often from complications of myocarditis, with associated arrhythmias, hepatic failure, and cerebral hemorrhage.
Diagnosis
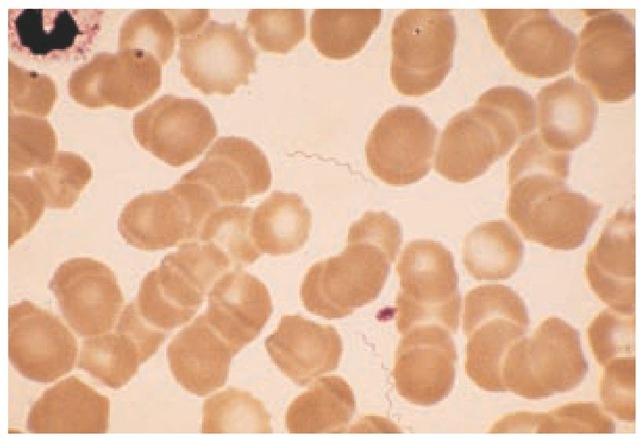
The febrile periods of illness are associated with high-level spirochetemia, and dark-field microscopy or Giemsa or Wright staining of blood smears can directly demonstrate Borrelia [see Figure 6]. Detection of spirochetes is improved with lysed thick smears or acridine orange staining.110,111 Spirochetes are rarely seen on blood smears obtained when the patient is afebrile. Culture is difficult and not readily available. Serology is of limited value, in part because of the antigenic variability of the organisms. Currently available assays target antigens common to other spirochetes and bacteria and do not have a high sensitivity. Sero-logic tests for both syphilis and Lyme disease may be positive.112
Figure 6 In this thin Wright stain of peripheral blood from a patient in the febrile stage of relapsing fever, several spirochetes can be seen.
Treatment
The Borrelia species that cause relapsing fever are susceptible in vitro to penicillins, tetracyclines, macrolides, cephalosporins, and chloramphenicol.113 LBRF can be successfully treated with a single oral dose of 500 mg of tetracycline. Young children and pregnant women may be treated with a single dose of erythromycin (500 mg).114 TBRF requires a 5- to 10-day course of antibiotics; shorter courses of treatment are associated with a higher rate of treatment failures. Tetracycline and erythromycin are felt to be equally effective. In patients with meningitis or encephalitis, a 14-day course of parenteral penicillin G, ceftriaxone, or cefotaxime is generally used. Jarisch-Herxheimer reactions are common within the first several hours after initiating antibiotic therapy and may be severe, with rigors, high fever, and hypotension. For this reason, it is recommended that all patients be kept under observation for several hours after the first dose of antibiotics.
Rat-Bite Fever
Rat-bite fever is a systemic febrile illness that results from infection with Streptobacillus moniliformis in North America and Europe and with Spirillum minus in Asia. These bacteria are transmitted to humans by the bite of a rat or other small rodent. Illnesses from the two organisms are similar, but each has unique clinical features [see Table 2].
Streptobacillus Moniliformis infection
S. moniliformis is a pleomorphic gram-negative bacillus that is frequently part of the nasopharyngeal flora of rats and other small rodents.115 Infection typically is transmitted by the bite or scratch of rats (also mice and squirrels) or by carnivores that prey on rodents. Despite the term rat-bite fever, approximately 30% of rat-bite fever cases occur in patients who have no history of being bitten by rats. Transmission may occur from handling rats at home and the workplace (pet shops, laboratories). Consequently, rat-bite fever should be in the differential diagnosis of unexplained febrile illness or sepsis in patients with any history of rat exposure. Illness can also result from oral ingestion of the organ-ism—for example, eating food that is contaminated with rodent droppings (Haverhill fever).
Clinical Features
The usual incubation period after a rodent bite is less than 10 days (range, 1 to 22 days). Onset of illness is abrupt, with fever, chills, headache, vomiting, and severe migratory arthralgias and myalgias.116 In contrast to S. minus infection, ulceration at the initial bite site and regional lymphadenopathy are usually absent with S. moniliformis infection. Within several days after the onset of the fever, a nonpruritic, maculopapular, petechial, vesicular or pustular rash develops on the extremities, with involvement of the palms and soles. An asymmetrical polyarthritis occurs in a significant number of patients; frank septic arthritis may develop. The large joints are most commonly involved (i.e., knees, ankles, wrists, shoulders, and hips).
Fever associated with S. moniliformis infection tends to subside after several days, even without specific therapy. The other clinical manifestations usually resolve over the next several weeks. Rare, potentially fatal complications include cardiac involvement (e.g., endocarditis, myocarditis, and pericarditis), meningitis, pneumonia, and abscesses in a variety of solid organs. Two cases of fulminant sepsis and death in previously healthy persons have been reported.118
Table 2 Characteristics of Rat-Bite Fever
|
Streptobacillus moniliformis Infection |
Spirillum minus Infection |
|
|
Incubation period |
10 days (1-22 days) |
1-4 wk |
|
Bite site |
Healed by the time symptoms appear |
Swollen, painful; may ulcerate |
|
Onset |
Chills, fever, joint symptoms |
Fever without joint symptoms |
|
Joint symptoms |
Asymmetrical polyarthritis common; occasional frank septic arthritis |
Usually absent |
|
Course |
Relapsing course uncommon |
Relapsing course common |
Diagnosis
S. moniliformis can be readily grown using enriched media. The mainstay of diagnosis is culture. In addition, pleomorphic gram-negative bacilli can be demonstrated on Gram stains of blood, joint fluid, and abscess aspirates. Antibody detection by ELISA and PCR of bacterial 16S ribosomal RNA in tissue samples have been described, but these tests are currently still limited to research centers.
Spirillum Minus infection
S. minus, a short, thick, gram-negative, tightly coiled spiro-chete, is the cause of rat-bite fever in Asia. Approximately 25% of rats in endemic areas are carriers of S. minus, and the major route of transmission of infection to humans is through the occurrence of rat bites.
Clinical Features
The original rat bite heals promptly, but 1 to 4 weeks later, the site of the bite becomes swollen, indurated, and painful, and it may ulcerate. In contrast to S. moniliformis infection, S. minus infection usually includes regional lymphadenitis. Headache, fever, chills, and malaise accompany the formation of the ulcer.121 A sparse, dusky-red maculopapular rash appears on the trunk and extremities in many cases. The severe arthritis and myalgias seen in S. moniliformis infection are rare in disease caused by S. minus.
Symptoms of S. minus infection subside after a few days but recur several days later. Without specific antibiotic therapy, fevers lasting 3 to 4 days recur at regular intervals between afebrile periods lasting 3 to 9 days. This relapsing course can persist for several months; in rare instances, fever relapses have occurred for a year or more. More serious complications include endocarditis, myocarditis, meningitis, conjunctivitis, hepatitis, and pleural effusions. Mortality without antibiotic therapy ranges from 6% to 10%.
Diagnosis
Leukocytosis is often present with S. minus, and up to 50% of patients will have a false positive test for syphilis. S. minus cannot be grown on artificial media. Initial diagnosis relies on visualization of spirochetes in blood, exudates, or lymph node tissue on culture staining or dark-field microscopy. Intraperitoneal inoculation of mice has been used to establish the diagnosis.121,122 No specific serologic test is available.
Treatment
Both S. moniliformis and S. minus infections are effectively treated with penicillin given for 10 to 14 days.123 Initial therapy for severely ill patients should be parenteral, but once the patient becomes stable, the course may be completed with oral penicillin or ampicillin. A Jarisch-Herxheimer reaction has been reported with initial therapy for S. minus infection. Oral tetracycline is appropriate for penicillin-allergic patients.