Complement and Disease
The complement system is a double-edged sword. Once unsheathed, it can attack in a robotic fashion. The pathophysiology of many inflammatory diseases involves the synthesis of autoan-tibodies and the presence of excessive quantities of immune complexes. If the host produces an antibody that reacts to a self-antigen (e.g., on an erythrocyte), the complement cascade becomes activated. Thus, just as complement can destroy a microbe, it may lyse an erythrocyte, opsonize a platelet, or disrupt a basement membrane. If immune complexes lodge in blood vessel walls in a particular tissue, they may activate complement to produce synovitis, vasculitis, dermatitis, or glomerulonephritis. Similarly, a powerful complement barrage may result from is-chemia-reperfusion injury as the alternative pathway elicits C3b deposition on the damaged tissue, which is regarded as foreign.
Complement component deficiencies, although rare, predispose to autoimmune diseases (e.g., SLE) and bacterial infections [see Table 8].17,18,27,28 Deficiencies of complement regulatory proteins allow excessive activation of complement cascades [see Table 7]. These conditions are usually inherited as autosomal recessive traits, with the exception of deficiencies of C1-Inh (which is an autosomal dominant trait) and properdin (which is X-linked). The effects of these conditions are predictable, because the affected person experiences a loss of function of the deficient protein and of all the proteins that would ordinarily follow in the cascade. Deficiencies of early components (e.g., C1q, C1r/C1s, C4, and C2) predispose to SLE, whereas deficiency of C3, MBL, or MAC components leads to recurrent bacterial infections.
C1-Inh deficiency causes hereditary angioedema, whose symptoms range in severity from a minor inconvenience to life-threatening laryngeal edema [see 6:XIII Urticaria, Angioedema, and Anaphylaxis].29 Deficiency of factor H may lead to uncontrolled AP activation on, and damage to, endothelial cells, resulting in hemolytic uremic syndrome or glomerulonephritis.
Acquired deficiencies of complement also predispose to illness. C1-Inh deficiency may occur as a result of excessive utilization of C1-Inh (usually because of a malignancy) or inactivation of C1-Inh by an autoantibody. An acquired deficiency of C3 may occur as a result of production of an autoantibody that binds and stabilizes the C3 convertase. In the AP, this antibody is called the C3 nephritic factor, whereas in the CP it is termed the C4 nephritic factor. Most patients with C3 nephritic factors are children; they may present with a combination of glomerulonephri-tis, partial lipodystrophy, and frequent infections with encapsulated bacteria.
Table 7 Complement Regulatory Proteins
|
Regulation Site |
Protein |
Tissue Distribution |
Function |
Disease Associated with Deficiency |
|
Initiation of complement cascade |
C1 inhibitor |
Plasma |
Inactivates C1r, C1s, and MASPs; a SERPIN |
Hereditary angioedema |
|
Convertases |
Factor I |
Plasma |
Cleaves C3b and C4b; requires a cofactor protein |
Infection (secondary to low C3 levels) |
|
Membrane cofactor protein |
Most cells |
Cofactor for cleavage of C4b and C3b |
Hemolytic-uremic syndrome |
|
|
Decay-accelerating factor |
Most cells |
Destabilizes C3 and C5 convertases |
None* |
|
|
C4b-binding protein |
Plasma |
Cofactor for cleavage of C4b; decays CP C3 and C5 convertases |
None* |
|
|
Factor H |
Plasma |
Cofactor for cleavage of C3b; destabilizes AP C3 and C5 convertases |
Hemolytic-uremic syndrome; glomerulonephritis |
|
|
Complement receptor type 1 (CR1) |
Blood cells |
Receptor for C3b and C4b; cofactor activity for C3b and C4b and decays C3 and C5 convertases |
None+ |
|
|
MAC |
S protein |
Plasma |
Blocks fluid-phase MAC |
Unknown |
|
CD59 |
Most cells |
Blocks MAC on host cells |
Paroxysmal nocturnal hemoglobinuria |
|
|
Other |
Anaphylatoxin inactivator |
Plasma |
Inactivates C3a, C4a, and C5a |
Hives |
*Too few cases of complete deficiency described to establish an association. +A complete deficiency has not been reported.
AP—alternative pathway
CP—classical pathway
MAC—membrane attack complex
MASPs—mannan-binding lectin-associated serine proteases
SERPIN—serine protease inhibitor
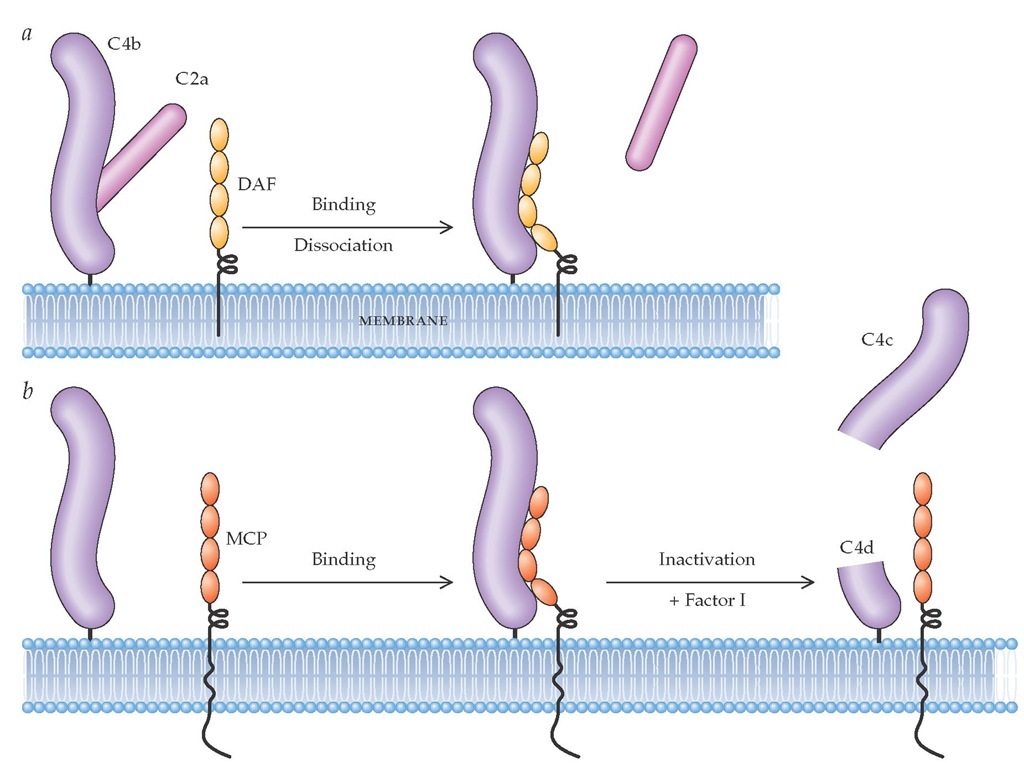
Figure 4 The membrane proteins decay-accelerating factor (DAF) and membrane cofactor protein (MCP) regulate the C3 and C5 convertases.35 These proteins function by disassembling the convertases (decay-accelerating activity), by facilitating proteolytic inactivation, or by both processes. In the classical pathway, the components of C3 convertase are the proteases C4b and C2a. Decay-accelerating activity (a) occurs when DAF binds C4b, displacing the C2a. Proteolytic inactivation (b) occurs when MCP, in concert with the serine protease factor I, cleaves C4b; this prevents C4b from interacting with newly formed C2a. The residual bound C4d has no known biologic activity. In the classical pathway, C5 convertase (which consists of a C4bC2a with an attached C3b) is similarly inactivated by decay-accelerating activity. Although C3 and C5 convertases in the alternative pathway have a different structure, they are disassembled in an identical fashion by DAF and MCP.
An acquired hematopoietic stem cell disorder produces paroxysmal nocturnal hemoglobinuria [see 5:IV Hemoglobinopathies and Hemolytic Anemias]. A mutation in a stem cell prevents expression of an enzyme needed to produce so-called greasy foot (i.e., glycolipid) proteins. As a result, blood cells are deficient in proteins that have this cellular anchor. In particular, deficiencies of CD59 and DAF predispose erythrocytes to complement-mediated hemolytic anemia.
Complement Measurement
Complement levels can be assessed using either antigenic or functional assays [see Table 9]. The former are easier to perform and are most commonly employed for measuring C3 and C4 levels. The total hemolytic complement (THC or CH50) assay measures activation of the entire CP by assessing the ability of the test serum to lyse sheep erythrocytes optimally sensitized with rabbit antibody. Interpretation of the results is rather straightforward [see Table 10]. Decreased C4 and C3 levels almost always indicate CP activation, whereas AP activation is indicated by normal levels of C4 but low levels of C3 (and factor B, if measured). All nine components of the CP (C1 through C9) are needed to obtain a normal result on the CH50 assay. A CH50 of 200 means the tested serum lysed 50% of the antibody-coated sheep erythrocytes when assayed at a dilution of 1:200. A similar assay, AH50, measures the total alternative pathway, with the target for lysis being unsensitized rabbit red blood cells. Less widely used tests include measurement of the anaphylatoxins C5a and C3a or activation fragments, such as C3d and Bb. Tests showing increased levels of these substances have the advantage of reflecting ongoing activation and are more sensitive. In addition, specialized laboratories can determine the functional and antigenic levels of each of the complement components and regulators.
Table 8 Clinical Manifestations of Complement Deficiency in the Activation Pathways
|
Pathway Involved |
Deficient Component |
Clinical Syndrome |
|
Classical pathway |
C1q |
SLE, infections* |
|
C1r/C1s |
SLE, infections* |
|
|
C4 |
SLE, infections* |
|
|
C2 |
SLE, infections* |
|
|
Lectin pathway |
MBL |
Infections |
|
Central component |
C3 |
Severe infections,* glomerulonephritis, SLE |
|
Membrane perturbation |
C5, C6, C7, C8, or C9 |
Neisseria infections |
|
Alternative pathway |
Properdin, factor D |
Neisseria infections |
*Typically, with commonly encountered pyogenic organisms.
MBL—mannan-binding lectin
SLE—systemic lupus erythematosus
Table 9 Assays for Complement Activation in Human Disease
|
Method |
Use |
Comments |
|
CH50 or THC |
Screen for a component deficiency or activation of classical pathway |
Functional assay; requires appropriate sample handling |
|
AP50 |
Screen for component deficiency or activation of alternative pathway |
Functional assay; requires appropriate sample handling |
|
Antigenic (ELISA, immunodiffusion, nephelometry) |
Standard method for C3, C4, factor B, C1-inhibitor, and MBL determinations |
Widely available, easy to perform, reliable, inexpensive |
|
Antigenic or hemolytic assay of individual components |
To further define a suspected deficiency |
Samples usually sent to laboratories specializing in complement assays |
|
Activation fragments C3a, C5a, Bb C1-INH; C1r/C1s C5b-9 (neoantigen) |
Additional tests to detect complement turnover |
More sensitive than static levels; sample collection technique important; often available through commercial laboratories; expensive |
|
C1-inhibitor function |
When clinical picture is consistent with HAE but C1-inhibitor levels by antigenic assay are normal or elevated |
15% of HAE patients have normal or elevated levels of a nonfunctional protein |
|
Immunofluorescence |
Demonstration of complement activation fragments in tissue |
C1q, C4, and C3 are most commonly studied in kidney and skin biopsy specimens |
|
Antiglobulin testing (nongamma Coombs) |
Demonstration of C3 fragments on erythrocytes |
Usual fragment detected is C3d |
AP50—alternative pathway equivalent of THC or CH50
ELISA—enzyme-linked immunosorbent assay
HAE—hereditary angioedema
MBL—mannan-binding lectin
THC—total hemolytic assay for classical pathway (also called CH50)
Evolving Therapeutic Strategies
Currently, no therapeutic agent is commercially available to block the deleterious effects of pathologic complement activa-tion.31-33 Two plasma-based therapies are undergoing clinical trials. A humanized monoclonal antibody to C5 adopts the straightforward strategy of blocking the function of a single component; specifically, it blocks cleavage of C5 by C5 conver-tases. A significant advantage of this approach is that recombi-nantly produced humanized monoclonal antibodies are already an established therapy in clinical medicine (e.g., anti-TNF). Re-combinant monoclonal antibodies have a relatively long half-life and, if humanized, are usually nonimmunogenic. However, it is unclear how much tissue damage in clinical syndromes results from the activation of C5 as compared with that caused by C3. An advantage of this therapeutic approach is that activation up to and including C3 remains intact.
A second compound in clinical trials is a solubilized version of CR1 (sCR1), which degrades C3b/C4b and decays C3/C5 convertases. Proof of principle has been established in animal models, as it has been for the monoclonal antibody to C5. Poten-tial barriers to the use of sCR1 in clinical practice include cost, lack of selectivity, the need for intravenous administration, and the potential for infectious complications.
Table 10 Interpretation of Complement Assays
|
|
Test Result |
|
|
|
|
|
|
Interpretation |
|
THC (units/ml) |
C4 (mg/dl) |
C3 (mg/dl) |
|
|
150-250 |
16-40 |
100-180 |
Normal range |
|
250 |
40 |
200 |
Acute-phase response |
|
100 |
10 |
80 |
CP activation |
|
100 |
30 |
50 |
AP activation |
|
< 10 or 0 |
30 |
140 |
Inherited deficiency or in vitro activation* |
|
50 |
< 8 |
100 |
Partial C4 deficiency or fluid-phase activation+ |
*In vitro activation is more common than an inherited deficiency state. The lack of activity (< 10 THC) in the setting of normal C4 and C3 antigenic levels suggests (1) an improperly handled sample, (2) cold activation (such as by cryoglobulins) after collection of the sample, or (3) homozygous component deficiency (most commonly C2 with a lupus presentation or, if a Neisseria infection is present, of an AP or membrane-attack-complex component).
+Detectable THC excludes a complete deficiency of C4. A partial C4 deficiency, such as of C4A, could give this result. Some types of immune complexes, especially cryoglobulins, and a deficiency of the C1-inhibitor (hereditary angioedema) also give this pattern. In these cases, measurement of C2 is often helpful: a low value suggests activation, whereas a normal value suggests an inherited, partial C4 deficiency. Also, C4A and C4B alleles can be assessed by commercial laboratories.
AP—alternative pathway
CP—classical pathway
THC—total hemolytic complement (also called CH50)
The search for a complement therapeutic has led to two unanticipated observations. First, complement activation contributes to tissue damage in ischemia-reperfusion injury syndromes such as myocardial infarctions and stroke; second, C5b-C9 and C5a bear responsibility for more of the tissue damage during complement activation than predicted.