Infantile-onset olivopontocerebellar atrophy
Infantile-onset olivopontocerebellar atrophy (IOSCA) has been described in a population isolate in Finland. Children with IOSCA present with ataxia, peripheral neuropathy,areflexia, athetosis, and Babinski signs early in the first decade of life. Other clinical features include speech impairment, hearing loss, optic atrophy, seizures, and learning deficits. IOSCA has been mapped to chromosome 10q24.20
|
Selected Internet Resources for Information on Ataxias |
|
HUGO (Human Genome Organization) Nomenclature Committee http://www.gene.ucl.ac.uk/nomenclature Names and symbols of human genes. |
|
OMIM (Online Mendelian Inheritance in Man) http://www3.ncbi.nlm.nih.gov/Omim |
|
A National Center for Biotechnology Information catalog of human genes and genetic disorders. |
|
GeneTests |
|
http://www.geneclinics.org Disease reviews, international directory of genetic testing laboratories and genetics and prenatal diagnosis clinics, educational materials. |
|
National Ataxia Foundation http://www.ataxia.org Hereditary ataxias patient support group. |
Other autosomal recessive ataxias
A significant proportion of childhood-onset or young-adult-onset ataxias remain unresolved at a molecular level. Koenig and coworkers have localized the gene for a syndrome comprising ataxia, neuropathy, cerebellar atrophy, and elevated creatine kinase levels to chromosome 9q33.3-34.3. They have also localized another disorder, involving ataxia, deafness, and optic atrophy, to chromosome 6p21-23.21 Other investigators have described a childhood-onset ataxia with deficiency of coenzyme Q10 levels in muscle.22
The combination of youth-onset ataxia and myoclonus or myoclonic seizures can be seen under a variety of circumstances. The term Ramsay Hunt syndrome is loosely applied to this entity. One disorder that has this combination of features is Unverricht-Lundborg disease, which has been linked to mutations in the cystatin B gene.23 Some patients with Ramsay Hunt syndrome may have defined diseases such as ceroid lipofuscinosis, sialidosis, and mitochondrial cytopathies.
The term early-onset ataxia with retained tendon reflexes was coined by the English neurologist Anita Harding in 1981 to describe children with progressive ataxia resembling FA, except for the retention of reflexes. Many of these patients are now known to harbor the FA mutation. In others, the genotype is still unknown. It is also known that a small minority of patients with the typical FA phenotype do not carry the FA mutation.24
Autosomal Dominant Ataxias
The autosomal dominant ataxias are genetically heterogeneous but share many clinical features. Therefore, precise identification of the underlying genotype on the basis of clinical features alone can be difficult. A clinical diagnosis is often helped by examining several affected family members to assess the degree of phenotypic variability in the same family. Autosomal dominant ataxias with a progressive course are labeled SCA, for spinocerebellar ataxia, followed by a number that denotes the specific gene locus involved. The SCA nomenclature is administered by the Human Genome Organization [see Sidebar Selected Internet Resources for Information on Ataxias].
Genetics and pathogenesis
Most of the autosomal dominant progressive ataxias elucidated to date are related to unstable expansions of repeated nucleotide sequences [see Table 2 and Figure 1]. A CAG expansion in the coding region of the gene occurs in SCA1, SCA2, SCA3, SCA6, SCA7, SCA17, and dentatorubral pallidoluysian atrophy (DRPLA).25-28 These are all examples of polyglutamine disorders, because CAG codes for glutamine. A CAG expansion in the promoter region of the gene occurs in SCA12.29 SCA8 has been related to a CTG expansion in an untranslated region of the gene30 and SCA10 to a pentanuc-leotide repeat expansion.
The CAG expansion disorders share certain features. The age at onset is typically inversely correlated with the number of CAG repeats in the expanded allele. In addition, the degree of expansion also correlates, to some extent, with rates of disease progression and some of the phenotypic features. Thus, larger expansions may result in more rapid loss of neuronal function and more widespread neuronal pathology. The normal alleles in these disorders are stable on intergenerational transmission. The expanded alleles are often unstable, and further expansion is more likely than contraction. Expansion of the repeat is more likely to occur with paternal inheritance than with maternal inheritance, and it often is of larger magnitude with paternal inheritance; this accounts for some of the anticipation (i.e., observed onset at a progressively earlier age in successive generations) in these disorders.
The genes with CAG expansions that are mutated in the autosomal dominant ataxias are widely expressed in neuronal and nonneuronal tissues. Such dominant ataxias are classified among the polyglutamine disorders because the CAG tract is translated into a polyglutamine stretch in the corresponding protein. There is increasing evidence that the polyglutamine disorders are related to the acquisition of an abnormal toxic property (gain of function) by the protein product of the mutated allele. Most of the gene products related to the CAG-expansion disorders are novel proteins of unknown function. Normally, many of these proteins are distributed diffusely in the cytoplasm and are variably present in the nucleus.
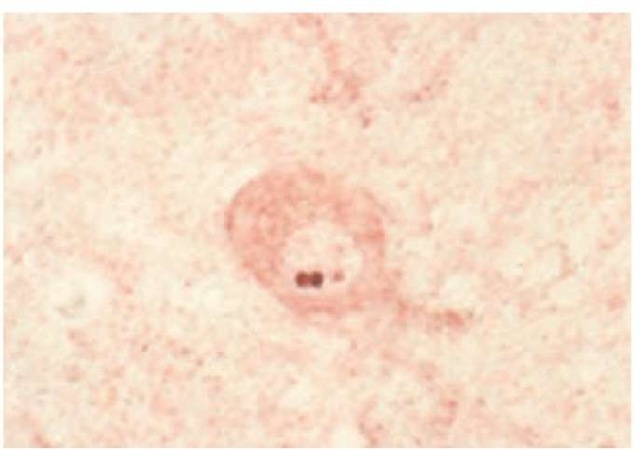
Figure 2 Aggregates of ataxin 3 are visible within the nucleus of a pontine neuron from a patient with Machado-Joseph disease.
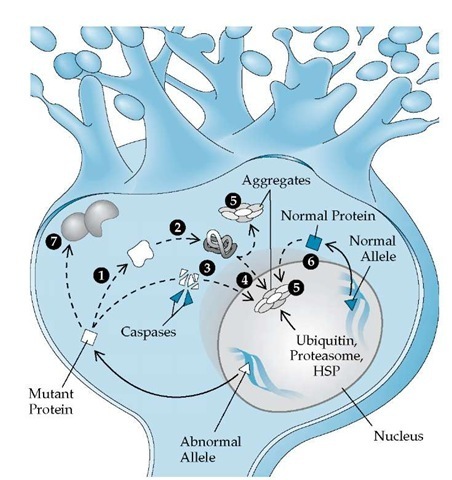
Figure 3 Schematic picture of some of the proposed pathogenic mechanisms in the polyglutamine ataxias. The abnormal allele (A) is translated into a protein with abnormal molecular weight (□). The mutant protein with an excess number of glutamine molecules may have an altered conformation (1), allowing misfolding (2) and perhaps cleavage by caspases (3). The entire mutant protein or cleaved fragments may enter the nucleus and form aggregates (4) or may aggregate in the cytoplasm (5). These aggregates often recruit ubiquitin, proteasome, and components of the heat shock protein (HSP) chaperone system, suggesting misfolding and attempts at proteolysis. The product of the normal allele may also be recruited into these aggregates (6). The presence of mutant protein in the nucleus may alter expression of other genes essential for neuronal function. Abnormal interaction of mutant protein with other proteins (7) may account for some of the specificity of neuronal loss.
Both the messenger RNA (mRNA) and the protein corresponding to the normal and the expanded alleles can be detected in the tissues of the affected persons. In contrast to the diffuse localization of the normal protein, neurons in patients with the disease tend to show aggregates of the mutated protein, mostly within neuronal nuclei [see Figure 2] but also in the cytoplasm and in the neurites. The role of these nuclear inclusions (NIs) in the pathogenesis of the dominant ataxias is debated [see Figure 3]. However, many observations have been made, such as the following25-27:
1. NIs can be identified in many transgenic and transfection models of the ataxias.
2. NIs can be dissociated from the pathology; thus, genetic engineering can abolish the presence of NIs without reducing the pathology; this has been documented for SCA1.
3. In SCA1, preventing nuclear entry of mutated protein can prevent pathology.
4. Truncated constructs of the gene resulting in fragments of the protein containing the polyglutamine tract can often be more toxic than full-length protein.
5. Caspases may be involved in the generation of such truncated version of the proteins in vivo.
6. NIs usually recruit many other proteins; the presence of proteins belonging to the heat shock protein (HSP) chaperone system suggests that the mutated polyglutamine protein assumes a misfolded configuration. Also, the presence of proteins belonging to the ubiquitin-proteasome pathway in the NI indicates attempts at removing the aggregated protein. Manipulation of both the HSP and the ubiquitin paths can alter the intensity of NI and sometimes the pathogenic severity.
7. The aberrant interaction of the mutant protein in the nuclear context may alter many essential nuclear functions. Many of the mutant proteins can bind transcription factors such as the cyclic adenosine monophosphate (cAMP) response element-binding protein (CREB)-binding protein (CBP). Others have RNA-binding activity. Such interaction may result in upregulation or downregulation of other important genes that can have pathogenetic implications.
The mechanism of disease in the nonpolyglutamine ataxias is still poorly understood. In the case of SCA8, the CTG expansion in the 3′ untranslated region of the gene may result in aberrant RNA activity that could be lethal to neurons.32 In SCA6 and the episodic ataxia (EA) syndromes, which are related to mutations in channel proteins, abnormal electrical properties of the mutated channel may have a role.33 Lastly, recent reports have identified point mutations in genes encoding protein kinase C-y (in SCA14), as well as fibroblast growth factor 14, as causes of progressive spinocerebellar ataxias.
Diagnosis
Clinical Features
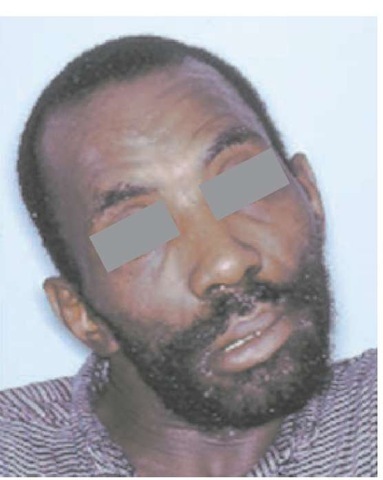
Patients with autosomal dominant ataxia present with progressive gait problems, dysarthria, and occasionally diplopia.36-38 In addition to having cerebellar signs, these patients exhibit a variety of other neurologic deficits. As the disease progresses, eye-movement abnormalities such as gaze paresis, lid retraction, and blepharospasm, as well as bulbar signs such as facial and temporal atrophy, facial fasciculations, and mild tongue atrophy, may occur. Many of the disorders are associated with upper motor neuron signs such as brisk deep tendon reflexes, spasticity, and extensor plantar responses. Some of the genotypes are associated with basal ganglia signs such as an akinetic rigid syndrome, dystonia [see Figure 4], and choreoathetosis. Involvement of peripheral nerves can be found in the form of distal sensory loss and areflexia, as well as amyotrophy, fasciculations, and cramps. Tremor resembling essential tremor and torticollis can occur in some cases. Cognitive changes, seizures, and myoclonus reflect cortical pathology. Evidence of retinal disease occurs in certain genotypes. Patients typically lose ambulation 10 to 15 years after onset and, at this stage, develop increasing dys-phagia; some may have urinary incontinence.
The EA syndromes cause intermittent episodes of ataxia; these are very short-lived (minutes) in EA-1 and longer (hours) in EA-2. Interictally, EA-1 can be associated with skeletal muscle myokymia and EA-2 with nystagmus. The EA-2 phenotype can be variable and may be similar to SCA6 and may be associated with migraine (both SCA6 and familial hemiplegic migraine are allelic to EA-2).
Figure 4 Dystonia in a patient with Machado-Joseph disease.
The various autosomal dominant ataxias are marked by phenotypic differences [see Table 4]. It should be stressed, however, that phenotypic features alone are often insufficient to permit diagnosis of the genotype in individual patients. Within specific genotypes, the phenotype may vary significantly. In many instances, this variance is related to the duration of disease; however, the variation in the number of nucleotide repeats from generation to generation can influence the phenotype. Thus, in all CAG repeat disorders, there is an inverse relationship between the age at onset and the size of the expanded allele. Other modifier genes that can influence the phenotype are currently the focus of considerable interest.
Table 4 Phenotypic Features That May Indicate a Specific Genotype in Autosomal Dominant Ataxias
|
Phenotypic Feature |
Disorders |
|
Age at onset |
Young adult: SCA1, SCA2, MJD |
|
Older adult: SCA6 |
|
|
Childhood: frequent in SCA7/DRPLA |
|
|
Degree of anticipation |
More in SCA7, DRPLA |
|
Benign course |
SCA6 |
|
Upper motor neuron signs |
SCAs 1, 7, and 8; MJD; rare in SCA2 |
|
Akinetic-rigid/Parkinson |
MJD, SCA2, SCA17 |
|
signs |
|
|
Chorea |
Prominent in DRPLA; late in SCA2, |
|
SCA1, MJD |
|
|
Action tremor |
SCA12, SCA16 |
|
Very slow saccades |
Early in SCA2, SCA7; late in SCA1, |
|
MJD; never in SCA6 |
|
|
Downbeat nystagmus |
SCA6, EA2 |
|
Generalized areflexia |
SCA2, SCA4, older adult-onset MJD |
|
Visual loss |
SCA7 |
|
Seizures |
SCA10, early-onset DRPLA, SCA7 |
DRPLA—dentatorubral pallidoluysian atrophy
EA—episodic ataxia
MJD—Machado-Joseph disease
SCA—spinocerebellar ataxia
Table 5 Normal and Expanded Range of Various Repetitive Nucleotide Sequences in Inherited Ataxias
|
Disease |
Repeat |
Normal |
Intermediate |
Expanded |
|
SCA1 |
CAG |
6-44 |
39-821 |
|
|
SCA2 |
CAG |
14-31 |
32-33 |
33-64 |
|
MJD |
CAG |
12-40 |
54-86 |
|
|
SCA6 |
CAG |
4-18 |
19-30 |
|
|
SCA7 |
CAG |
4-27 |
28-36 |
37-200 |
|
SCA8 |
CTG |
16-91 |
107-1272 |
|
|
SCA10 |
ATTCT |
10-22 |
1,000-4,500 |
|
|
SCA12 |
CAG |
< 29 |
66-78 |
|
|
SCA17 (TBP gene) |
CAG |
25-44 |
50-63 |
Note: Pathogenic and nonpathogenic alleles of the same size can be distinguished by the presence of CAT interruptions in the latter. The test for CTG expansion in SCA8 has to be interpreted with caution; whether the CTG expansion is the specific cause for ataxia in SCA8 has been debated, because in many instances this expansion has been found in patients with unrelated disorders. Yet at least in one large family, the repeat expansion was significantly associated with ataxia. MJD—Machado-Joseph disease SCA—spinocerebellar ataxia TBP—TATA-binding protein
Laboratory Studies
Imaging studies typically show either isolated cerebellar atrophy (in SCA6 and other disorders with a so-called pure cerebellar presentation) or pontocerebellar atrophy (in SCA1, SCA2, SCA3, SCA7). Individual genotypes cannot be distinguished by imaging studies alone. T2-weighted images may show increased signal density along the pontocerebellar fibers.
Nerve conduction studies often show evidence of an axonal neuropathy in some disorders (SCA 1 through 4). Brain stem evoked potentials are abnormal in the disorders associated with brain stem pathology. Abnormal results on electro-retinography can antedate visual loss in patients with SCA7.
Molecular tests are now available for SCA1, SCA2, SCA3, SCA6, SCA7, SCA8, SCA10, SCA12, SCA17, and DRPLA [see Table 5]. Gene tests are possible for the EA syndromes but involve sequencing or similar tests that are technically more difficult; these diseases are better diagnosed by clinical and biochemical parameters.
Differential Diagnosis of Inherited Ataxias
In patients presenting with progressive ataxia, a number of acquired disorders need to be excluded by appropriate imaging and laboratory studies. These disorders include vascular problems such as infarcts or malformations; tumors; demyelinating disease; paraneoplastic cerebellar degenerations; hypothyroidism; and cerebellotoxic disease, such as that related to alcohol, antiepileptic drugs, and cytosine arabinoside. Other possible immune etiologies for ataxic disorders have been proposed, including antibodies against glutamic acid decarboxylase and gliadin. Lastly, in a substantial number of patients, a definitive genetic or environmental cause cannot be established. Such patients are diagnosed as having late-onset sporadic or idiopathic ataxia.
In young adults or children, genetically determined metabolic errors may have ataxia as a dominant clinical feature. These disorders need to be excluded when appropriate. Examples of such diseases are Wilson disease, cerebro-tendinous xanthomatosis, abetalipoproteinemia, some of the leukodystrophies and gangliosidoses, sialidosis, ceroid lipo-fuscinosis, carbohydrate-glycoprotein deficiency syndromes, and giant axonal neuropathy.
Management of Inherited Ataxias
Currently, no specific interventions are available for most of the inherited ataxias. The possible treatment strategies in FA have been discussed (see above). When specific biochemical defects are identified, these can possibly be corrected—for example, with vitamin E supplements in AVED, other vitamin therapy, or dietary manipulations. The episodic ataxias (EA-1 and EA-2) often respond to acetazolamide.
The progressive ataxias are treated by rehabilitative measures such as assistive equipment, home modification, dysphagia treatment, and physical therapy. Several symptoms may need to be treated, including depression, anxiety, and pain.
Genetic counseling is important. In the dominant ataxias, offspring of affected persons have a 50% risk of developing the disease. When the molecular defect in the family is known, predictive testing can be performed. The guidelines used for Huntington disease are usually adopted for this purpose.