The inherited ataxias are disorders that cause progressive imbalance as a result of pathology in the cerebellum and its various connecting pathways. Ever since Nicholas Friedreich’s initial recognition, more than 100 years ago, that ataxia may result from genetic mechanisms, clinicians and investigators have been struck by the variability and complexity of the clinical, neuropathologic, and genetic aspects of these disorders. In addition to their difficulties with balance, persons with inherited ataxias often exhibit a variety of clinical signs that indicate dysfunction in the basal ganglia, the upper motor neuron, the oculomotor and other brain stem nuclei, the anterior horn cells, and the dorsal root ganglia. In some instances, vision loss and cognitive decline may occur as well.
Within the past decade, molecular genetic studies have identified specific gene mutations in many inherited ataxias and have localized the cause of others to specific chromosomal loci. This has led to the following advances:
1. Classification of these disorders on the basis of genotype.
2. Ability to identify the precise genotype in many patients.
3. Availability of predictive and prenatal diagnostic studies.
4. An understanding of some of the phenotypic variability related to mutations in the same gene.
5. Growing knowledge about the pathogenetic mechanisms of neuronal loss and clinical disease in the setting of specific gene mutations.
6. Early attempts at specific drug therapies in some of the diseases, based on this evolving knowledge.
Genotypic Classification
Within the past dozen years, the surprising genetic heterogeneity of the inherited ataxias has been amply documented. Some autosomal recessive and autosomal dominant ataxias have been defined at a molecular level [see Tables 1 and 2]. However, some early-onset ataxic syndromes remain poorly understood at a genetic level [see Table 3]. In addition, approximately a third of all autosomal dominant ataxias still have undiscovered gene loci. There are also ataxic disorders that have either mitochondrial or X-linked inheritance patterns.
Autosomal Recessive Ataxias
Friedreich ataxia
Friedreich ataxia (FA) is the most common type of re-cessively inherited ataxia; it has a prevalence of 2 x 10-5.1
Genetics and Pathogenesis
The mutation in FA is an unstable expansion of a GAA trinucleotide repeat in the first intron of the gene X-25, located on chromosome 9q [see Figure 1]. Normal alleles have fewer than 40 GAA repeats; over 80% of these are short normal alleles, which carry six to 10 repeats.1 Long normal alleles, which carry more than 12 repeats, may serve as a reservoir for expansion into pathogenic alleles; they appear to be confined to Indo-Caucasian populations. Enlarged alleles carry from 66 to over 1,000 repeats.2-5 Most patients with FA are homozygous for the trinucleotide expansion; fewer than 5% of patients with typical FA, however, carry only one expanded allele coupled with a point mutation in the second allele.6
The presence of the expanded GAA sequence results in reduced transcriptional and translational efficiency of the gene, which leads to a deficiency of frataxin, a nuclearly encoded mitochondrial protein. This effect appears to be related to so-called sticky DNA, an unusual DNA configuration induced by the expansion.7 In yeast, lack of the yeast frataxin homologue (YFH) causes a variety of disturbances, including reduced respiratory efficiency of mitochondria, loss of mitochondrial DNA, excess intramitochondrial iron, and increased susceptibility to oxidative stress.8,9 A similar process may occur in human tissues. Iron accumulation has been noted in cardiac myocytes at autopsy and in the cerebellar dentate nuclei on magnetic resonance imaging.10 Endomyocardial biopsies from patients with FA have shown a reduction in complexes I, II, and III, as well as levels of the enzyme aconitase. These enzymes share a common feature: the presence of iron-sulfur (Fe-S) clusters. Fe-S cluster proteins are especially susceptible to oxidative stress, which can be triggered by excess iron. It is also possible that the deficiency of Fe-S cluster enzymes may be related directly to frataxin deficiency.
Table 1 Autosomal Recessive Ataxias with Known Gene Loci
|
Disease |
Gene Locus |
Gene |
Mutation |
|
Friedreich ataxia |
9q13-21.1 |
X25 |
GAA expansion |
|
Ataxia-telangiectasia |
11q22-23 |
ATM |
Point mutations/deletions |
|
Ataxia with isolated vitamin E deficiency |
8q |
a-TTP |
Point mutations |
|
Autosomal recessive ataxia of Charlevoix-Saguenay (ARSACS) |
13q11 |
SACS |
Point mutations |
|
Ataxia with oculomotor apraxia |
9p13 |
Aprataxin |
Point mutations/deletions/insertions |
|
Ataxia, neuropathy, high a-fetoprotein |
9q33-34 |
Unknown |
Unknown |
|
Infantile-onset olivopontocerebellar atrophy (IOSCA) |
10q24 |
Unknown |
Unknown |
|
Ataxia, deafness, optic atrophy |
6p21-23 |
Unknown |
Unknown |
|
Unverricht-Lundborg disease |
21q |
Cystatin B |
Repeat expansion |
|
Ataxia-telangiectasia-like disorder (ATLD) |
11q21 |
MRE11 |
Point mutations |
|
Spinocerebellar ataxia with axonal neuropathy (SCAN-1) |
14q31 |
TDP1 |
Point mutations, insertions, deletions |
Table 2 Autosomal Dominant Ataxias with Known Gene Loci
|
Disease |
Locus |
Gene |
Mutation |
|
SCA1 |
6p23 |
Ataxin1 |
CAG expansion |
|
SCA2 |
12q23-24.1 |
Ataxin2 |
CAG expansion |
|
MJD (SCA3) |
14q21 |
Ataxin3 |
CAG expansion |
|
SCA4 |
16q24ter |
* |
* |
|
SCA5 |
11p11-q11 |
* |
* |
|
SCA6 |
19p |
CACNA1 |
CAG expansion |
|
SCA7 |
3p21.2-12 |
Ataxin 7 |
CAG expansion |
|
SCA8 |
13q21 |
Ataxin 8 |
CTG expansion |
|
SCA10 |
22q13 |
Ataxin 10 |
ATTCT expansion |
|
SCA11 |
15q14-21.3 |
* |
* |
|
SCA12 |
5q31-33 |
PPP2R2B |
CAG expansion |
|
SCA13 |
10q13.3-13.4 |
* |
* |
|
SCA14 |
19q13.4 |
PKCy |
Point mutations |
|
SCA16 |
8q23-24.1 |
* |
* |
|
SCA17 |
6q21ter |
TBP |
CAG expansion |
|
SCA18 |
7q31-32 |
* |
* |
|
SCA19 |
1p21-q21 |
* |
* |
|
SCA21 |
7p21 |
* |
* |
|
SCA22 |
1p21-q23 |
* |
* |
|
SCA23 |
20p13 |
* |
* |
|
DRPLA |
12p |
Atrophin |
CAG expansion |
|
EA-1 |
12p |
KCNA1 |
Point mutations |
|
EA-2 |
19p |
CACNA1 |
Point mutations |
*Unknown
DRPLA—dentatorubral pallidoluysian atrophy
EA—episodic ataxia
MJD—Machado-Joseph disease
TBP—TATA-binding protein
SCA—spinocerebellar ataxia
PKCy—protein kinase Cy
Diagnosis
Clinical features The onset of symptoms in patients with FA is usually in the first or second decade of life but may be delayed to the third decade or later. The classic descriptions of the disease include progressive gait ataxia with onset before 25 years of age, loss of deep tendon reflexes, and proprioceptive loss in the limbs—all signs of major early pathology in the dorsal root ganglion cells and their peripheral sensory processes. Other signs include dysarthria, extensor plantar responses, and oculomotor abnormalities such as square-wave jerks. Muscle atrophy, weakness, and dysphagia occur late in the disease. Tremor, vision loss, and hearing loss may occur in a few patients. About 30% to 50% of patients develop symptomatic heart disease, including hypertrophic cardiomyopathy. Diabetes occurs in 10% of patients, and skeletal deformities, such as scoliosis, are frequent.
The FA GAA expansion has been shown to be associated with a more variable phenotype than the classic features (see above).2-5 For example, the onset may be at a much older age (up to the early 50s). In addition, ataxia associated with preserved and, occasionally, brisk reflexes can occur. These phenotypes have been labeled late-onset Friedreich ataxia (LOFA) and Friedreich ataxia with retained reflexes (FARR), respectively. Age at onset is inversely correlated with the size of the GAA expansion, especially that of the smaller allele. Patients with heterozygous expansion coupled with a point mutation also often have atypical phenotypes with slower disease progression, especially when the point mutation occurs in the amino-terminal half of the protein.6
Laboratory tests One laboratory test that is of value is the FA mutation analysis. More than 95% of patients with typical FA will have a homozygous GAA expansion, but a heterozygous expansion is also diagnostic; in such cases, it can be presumed that the unexpanded allele has a point mutation. If the clinical picture is atypical, however, a heterozygous expansion may simply reflect an incidental carrier state. The FA mutation analysis is also indicated in almost all cases of recessive or sporadic ataxia of childhood or adult onset that cannot be readily categorized otherwise. Two other useful laboratory tests are nerve conduction tests, which show a predominantly sensory neuropathy, and brain imaging, which shows spinal cord atrophy with a relatively preserved cerebellum.
Differential Diagnosis
The phenotype of typical childhood-onset FA is usually not difficult to recognize. The major differential diagnoses include Charcot-Marie-Tooth disease and pure sensory neuropathies, as well as certain metabolic errors that can mimic FA disease, such as vitamin E deficiency.
Management
Laboratory studies have shown a beneficial effect of the coenzyme Q analogue idebenone on iron-induced oxidative damage. Idebenone has also been reported to have a therapeutic effect on the cardiomyopathy of FA.11 More extended studies are in progress. A combination of coenzyme Q10 (400 mg daily) and vitamin E (2,100 units daily) has been shown to improve cardiac and skeletal muscle bioenergetics in FA patients, as assessed by magnetic resonance spectroscopy.12
Because the specific therapy for FA is still in an experimental stage, symptomatic management of the disease still requires attention. This includes appropriate rehabilitation measures; monitoring and treating the systemic features, such as cardiomyopathy and diabetes; surgical correction of skeletal deformity when appropriate; and other types of symptom management. In end-stage FA, appropriate palliative measures need to be taken.
Table 3 Childhood or Young Adult-Onset Ataxias with Ill-Defined Genetic Abnormalities
|
Disease |
Clinical or Laboratory Feature |
|
Early-onset ataxia with retained deep tendon reflexes |
Some have FA/ARSACS mutation; others unknown |
|
Ataxia with hypogonadism (Holmes ataxia) |
Primary or secondary hypogonadism |
|
Ataxia with coenzyme Q10 deficiency |
Low coenzyme Q10 levels in muscle |
|
Ataxia with myoclonus |
MtDNA mutations, sialidosis, ceroid lipofuscinosis |
FA/ARSACS—Friedreich ataxia/autosomal recessive ataxia of Charlevoix-Saguenay
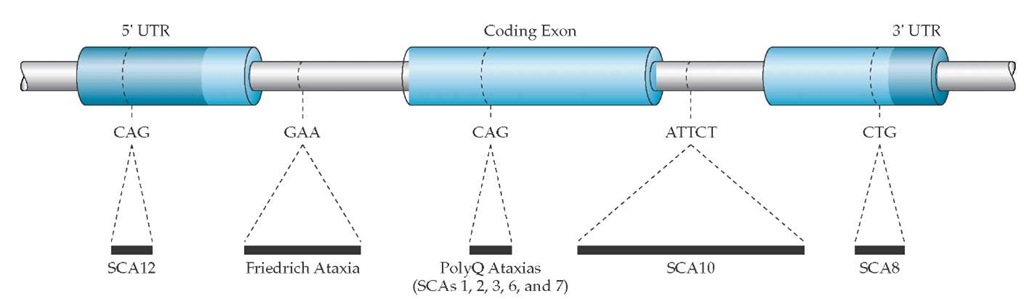
Figure 1 Diagrammatic representation of the type of repeat expansions associated with ataxias. (SCA—spinocerebellar ataxia; UTR—untranslated region)
FA families often need genetic counseling. The risk in siblings of patients is 25%. The carrier frequency in Indo-Cau-casian populations has been estimated to be approximately one in 100.
Ataxia with isolated vitamin e deficiency
Ataxia with isolated vitamin E deficiency (AVED) is a recessively inherited disorder that resembles FA, with childhood onset of ataxia, areflexia, and proprioceptive loss. Vitamin E levels are low, and vitamin E supplementation may arrest disease progression. Mutations in the a-tocopherol transfer protein gene (TTP) have been identified in AVED.13 The phenotype can vary depending on the type of mutation and the degree of residual TTP activity; older age at onset and retention of tendon reflexes cannot exclude AVED. Thus, measurement of vitamin E levels is indicated in any case of sporadic or autosomal recessive ataxia with onset during childhood or the young-adult years.
Ataxia-telangiectasia
Ataxia-telangiectasia (AT) typically manifests itself early in the first decade of life as increasing gait ataxia. Neurologic signs that evolve during the first decade include hypotonia, choreoathetosis, areflexia, and a characteristic oculomotor disorder that is associated with an impaired ability to generate saccades, which necessitates head thrusts to move the eyes. The typical telangiectasia appears in children at about 5 years of age and can be found over the conjunctiva, eyelids, and antecubital and popliteal fossae. These children have a high risk of malignancies, especially lymphomas. Measurement of altered radiation sensitivity and elevated a-fetoprotein levels in serum are useful to confirm the diagnosis.
The underlying gene defect in AT involves the AT mutated (ATM) gene, which is located on chromosome 11q. To date, AT has been linked to over 300 mutations in the ATM gene14; this knowledge has made prenatal molecular diagnosis of AT possible in some families The protein product of ATM appears to function as a protein kinase and is activated by ionizing radiation, with subsequent activation of cell cycle checkpoints. Defective checkpoint function may underlie some of the hematologic and immunologic phenotypes of AT. The molecular basis of Purkinje cell degeneration in AT is still poorly understood.
Because there are numerous mutations scattered over the ATM gene, DNA diagnosis of AT is more cumbersome than the clinical and biochemical diagnosis. Management of AT involves the care of its many systemic manifestations, such as neurologic deficits and increased susceptibility to infections and malignancies.
Autosomal recessive ataxia of charlevoix-saguenay
This spastic ataxia with onset in early childhood, which was first described in a population in the Charlevoix-Saguenay province of Quebec, has recently been linked to mutations in the Sacsin gene, which is located on chromosome 13.15 Ataxias in families in other parts of the world have been linked to the same locus, but the worldwide prevalence of this mutation in patients with non-FA childhood ataxia remains to be determined.
Autosomal recessive ataxia with oculomotor apraxia
This childhood-onset ataxia is marked by oculomotor apraxia, areflexia, and cerebellar atrophy. The serum albumin level is low, and the serum cholesterol level may be elevated. Mutation in a gene encoding a widely expressed protein-sharing homology with the histidine-triad proteins (HIT) and with the DNA-binding zinc-finger proteins has been identified in this disease.16,17 This gene may be involved in DNA single-strand break repair.
Other disorders of dna break repair
Interestingly, additional abnormalities of DNA single- or double-strand break repair have been shown to cause autosomal recessive ataxias. These include ataxia-telangiectasia-like disorder (ATLD) related to mutations in the gene mRE11 and spinocerebellar ataxia with axonal neuropathy (SCAN1) caused by a mutation in the gene encoding tyrosyl-DNA phosphodiesterase-1 (Tdp1).18,19 Thus, the list of disorders of DNA break repair that have been noted to cause ataxia include AT, ataxia with oculomotor apraxia, ATLD, SCAN1, Cockayne syndrome, and xeroderma pig-mentosum.