Diphtheria
Diphtheria derives its name from the Greek word diphtheria, meaning leather hide, which is the character of the pharyngeal membrane that is a hallmark of this disease. Once the cause of major epidemics in Europe and the United States, diphtheria was nearly eradicated by medical discoveries in the late 1800s and early 1900s. Conditions in both developing and developed countries have led to several recent outbreaks and have raised concerns that diphtheria may again become a serious public health problem.
Microbiology
Diphtheria is caused by Corynebacterium diphtheriae, a gram-positive rod with club-shaped swellings at each end. It is non-motile and is capable of growing on blood agar plates, causing a narrow band of hemolysis. Most strains produce a highly lethal exotoxin, whose production requires the presence of a bacteriophage that carries a specific determinant for toxin production. The organism produces characteristic metachromatic granules that stain bluish purple with methylene blue. Their snapping division results in angular and palisade arrangements of the cells on smear that frequently take on the appearance of Chinese lettering.1
Etiology and epidemiology
Humans are the only natural host for C. diphtheriae. The organism is most commonly spread by upper respiratory tract droplets. Persons incubating the disease, those convalescing from the infection, and healthy carriers can spread the disease to others through close contact. Asymptomatic carriage can persist for years within a population. For example, in Russia the clonal strain responsible for a large diphtheria outbreak was carried asymptomatically in the population for at least 5 years before the epidemic.2 Spread is more common during the colder months of the year, when people tend to be crowded indoors.3 Persons with C. diphtheriae skin lesions can also serve as reservoirs for the organism, and contamination of the environment tends to be greater from skin infection than from upper respiratory tract in-fection.4 Because the organism can survive as long as 6 months in fomites and dust, these objects and particles can serve as vehicles for transmission.
Diphtheria toxoid immunization prevents the serious complications of diphtheria, alleviating the clinical manifestations of the disease by blocking the toxin’s ability to enter cells. Immunization reduces local colonization of the nasopharynx with toxin-producing strains by reducing their survival advantage. In the United States, with the advent of widespread vaccine administration in the 1940s, the carrier state has dropped to very low levels, and the incidence of diphtheria has steadily declined. Before the initiation of the vaccination program, as many as 125,000 cases and 10,000 deaths were reported annually in the United States. Subsequently, the incidence declined to zero to five cases a year from 1980 to 1990. History indicates that diphtheria outbreaks occur in cycles that may include quiescent periods of up to 100 years. Therefore, the downturn in incidence may represent a normal cycle rather than herd immunity. Occasional outbreaks have been observed in Texas, Washington, and South Dakota.5 Two outbreaks were associated with urban alcoholics who practiced poor hygiene and lived in crowded environments; a third occurred in a Native-American community. Day care centers can also serve as a site for the spread of diphtheria. Outbreaks in Russia (15,211 cases) and the Ukraine (2,987 cases) are thought to have resulted from the decreased immunization of infants and children, as well as from waning immunity to diphtheria in older people.6 The resurgence of diphtheria in developed countries raises concern that reductions in the immunization of young children and the failure to revaccinate older adults could lead to a worldwide increase in the incidence of this very serious disease. The finding that only 30% of persons older than 70 years in the United States have protective antibody levels to diphtheria further emphasizes the potential for resurgence.7
Pathogenesis
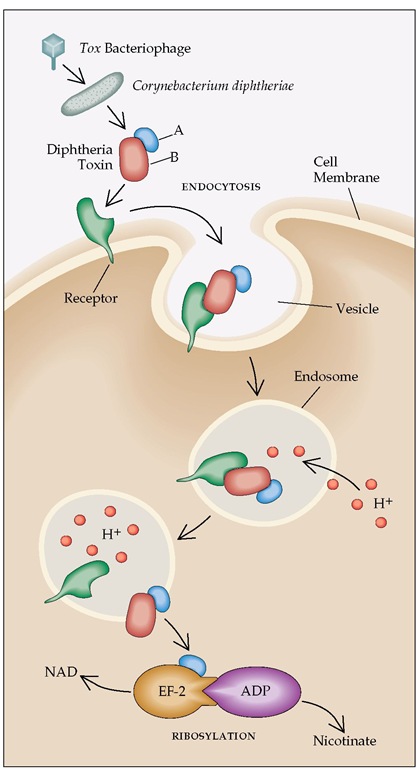
C. diphtheriae attaches to mucosal surfaces, particularly in the nasopharynx. Ocular and genital mucosae are less often infected. The skin has become an increasingly common site of infection, particularly in the United States. The diphtheria bacillus rarely invades living tissue but generally remains in superficial layers of the mucosa and skin. The major manifestations of this often serious disease result from production of a potent exotoxin. When iron concentrations are low, diphtheria bacilli possessing the corynebacteriophage containing the tox gene produce high concentrations of the toxin. This protein has a molecular weight of 62,000 and consists of two major fragments, designated A and B. Fragment B binds to a specific host cell membrane receptor, resulting in endocytosis of the entire molecule. Fragment B then forms a membrane channel that allows fragment A to enter the cell cytoplasm, block protein synthesis, and induce cell death within hours [see Figure 1].8 Antitoxin antibody can neutralize toxin adsorbed to cells or in the extracellular fluid. However, once the toxin penetrates cells, its toxic effects are irreversible.
In the host, the cytotoxic effects of the toxin are most marked in regions where bacterial growth is heavy and toxin concentrations are highest. Tissue necrosis is associated with an inflammatory response, leading to the formation of an adherent membrane that is greenish-gray to black and consists of fibrin, necrot-ic tissue, lymphocytes, polymorphonuclear leukocytes, erythrocytes, and bacterial colonies. All cells are susceptible to the lethal effects of the toxin; however, the heart, kidneys, and nervous system are injured most often.
Diagnosis
Clinical Manifestations
The incubation period for respiratory diphtheria is generally 2 to 4 days but can be as long as 7 days. Infection is usually associated with low-grade fever (approximately 38° C [100.4° F]); early in the infection, systemic complaints often are minimal. Symptoms depend on the location and duration of the infection before treatment. Concentrations of bacteria increase over time, resulting in the release of increasing amounts of the cytotoxic exotoxin.
Early recognition and treatment are therefore critical for reducing the complications associated with toxin dissemination.
Respiratory tract infection Pharyngeal infection, the most common form of diphtheria, presents as a sore throat and malaise. Symptoms may be mild in vaccinated patients, whereas unvaccinated patients tend to have more severe disease. Initially, diphtheria pharyngitis results in the development of a patchy white exudate that can be readily removed and is indistinguishable from group A streptococcal or viral pharyngitis. However, as the toxin begins to cause cell necrosis, a thin membrane begins to form that progressively thickens and spreads over the tonsils, posterior pharynx, and uvula [see Figure 2]. Initially, the membrane is white and smooth but later becomes gray, with patches of green and black necrosis. Particularly in children, the membrane can spread from the posterior wall up into the nose or down to the larynx or even the tracheobronchial tree.9 As the membrane spreads, it can interfere with normal airflow and lead to suffocation. Such extensive spread is generally associated with increased release of exotoxin, resulting in myocardial and neurologic complications and greatly increasing mortality. Laryngeal involvement results in hoarseness and may serve as a warning of impending respiratory compromise.10 Less often, the membrane may be limited to the nasopharynx, causing a serosan-guineous nasal discharge. Such limited involvement is less likely to be associated with generalized toxicity. Patients who have no membrane, but only pharyngeal erythema, almost always have milder, uncomplicated disease. Other clinical manifestations include tachycardia, cervical adenopathy, leukocytosis, and pro-teinuria. Development of the classic bull neck, the result of massive adenopathy, is now uncommon.
Cutaneous diphtheria Although outbreaks of cutaneous diphtheria were originally described primarily in tropical areas, outbreaks over the past 3 decades have been reported in the Pacific Northwest, Midwest, and southern United States. Cutaneous infection may result in greater environmental contamination than respiratory diphtheria and may carry a greater risk of spread to others. Unlike pharyngeal diphtheria, which usually develops in the winter months, cutaneous infections tend to peak in the late summer and early fall. Most cases are reported among residents of Seattle’s Skid Road district, who practice poor hygiene and often have preexisting sores.11 Skin infections have also been described in young schoolchildren. The classic punched-out ulcerative lesion often found in the tropics is rare in temperate climates. When the lesion does develop, it generally begins as a pustule that progresses to an ulcer with a gray-brown membrane at its base. More commonly, diphtheria superinfects preexisting skin lesions, including traumatic breaks in the skin, insect bites, ecthyma, and impetigo. In association with C. diphtherias, other skin pathogens, particularly Staphylococcus and Streptococcus, are found on culture. These infections tend to be indolent and are rarely associated with signs of intoxication, probably because cutaneous infections induce high levels of antitoxin antibody.
Other sites of infection Less often, C. diphtheriae infects the conjunctiva, eye, ear, and vagina. In Seattle, ocular infections in Skid Road residents sometimes accompanied skin involvement.
Complications
Systemic complications are caused by release of the diphthe-ria exotoxin, which predominantly damages the heart and nervous system.
Figure 1 Mechanism of action of diphtheria toxin. Diphtheria toxin production is encoded by a bacteriophage carrying the tox gene that gains entry into Corynebacterium diphtheriae. The toxin consists of an A fragment and a B fragment. The B fragment binds to the receptor (heparin-binding epidermal growth factor precursor) on the cell surface, and the whole molecule is then taken into the cell by endocytosis. In the closed environment of the endosome, acidification occurs (H+). The low pH level causes the B region to unfold and form a membrane channel, allowing the A domain to pass through the membrane into the cytoplasm. The disulfide bond linking the A and B regions is reduced, and the A subunit is then freed to bind to ADP-ribosylate elongation factor-2 (EF-2). Ribosylation interferes with the ability of EF-2 to add amino acids to a peptide chain, blocking protein synthesis and causing cell death. (A—A fragment; B—B fragment; NAD—nicotinamide adenine dinucleotide; ADP—adenosine diphosphate)
Myocarditis Subtle evidence of myocarditis is found in up to two thirds of patients with respiratory diphtheria.12 Clinically significant cardiac dysfunction is observed in 10% to 20% of patients. The severity of cardiac compromise correlates with the extent and severity of respiratory tract involvement. Cardiac toxic-ity generally occurs within 1 to 2 weeks after onset of the illness, often when pharyngeal symptoms are improving. The electrocardiogram generally reflects the severity of myocardial involvement and should be followed closely in all cases. ST segment and T wave changes and first-degree heart block are found in less severe disease, whereas left bundle branch block and AV block are associated with high mortality. In addition to damaging the Purkinje system, the toxin causes necrosis of cardiac muscle cells that can result in acute heart failure and circulatory collapse. A poor outcome is more likely in patients with extensive pharyngeal membrane and an aspartate aminotransferase level above 80 IU/L.13 Recovery in more severe cases of myocarditis can result in normalization of the ECG; however, patients usually sustain permanent injury to the myocardium.
Neurologic toxicity Neurologic complications occur in approximately 10% of respiratory cases. As with myocardial involvement, the likelihood of neurologic involvement correlates with the severity of the respiratory infection. Symptoms develop 10 to 28 days after the onset of respiratory complaints. Two types of neuropathy are seen. The first, cranial nerve involvement, is generally limited to the glossopharyngeal and vagus nerves, resulting in difficulty swallowing, aspiration, nasal re-gurgitation, and loss of the gag reflex. Less often, oculomotor and facial nerves become impaired. The second type of neuropathy tends to occur somewhat later in the course of the illness and resembles Guillain-Barre syndrome. These patients have quadriparesis associated with hyporeflexia. Degeneration of myelin sheaths and axon cylinders is observed in biopsy specimens. After treatment of the infection, slow but complete neurologic recovery ensues.
Physical Examination and Laboratory Tests
Prompt recognition and treatment of respiratory diphtheria are critical for preventing complications and mortality. In the early stages, diphtheria pharyngitis mimics group A streptococ-cal pharyngitis and mononucleosis. As diphtheria progresses,unlike in pharyngitis and mononucleosis, the exudate changes, becoming darker and forming a membrane that cannot be removed without causing bleeding. Neurologic abnormalities, such as ninth and 10 th nerve deficits or ECG changes, should also alert the clinician to the possibility of diphtheria. The microbiology laboratory staff must be notified of the possibility of C. diphtheriae because normal throat flora usually overgrow on blood agar plates. Nonnutritive, moist, reducing transport medium is helpful in preventing the overgrowth of competitors, and samples need to be inoculated on Loffler and tellurite media for proper identification. Assays for diphtheria toxin production also need to be performed.
Figure 2 Diphtheritic membrane extending across the uvula in a 47-year-old woman. Neck edema also has developed.
Treatment
All patients with a clinical diagnosis of respiratory diphtheria should be hospitalized and isolated, because the course of illness is unpredictable. Rapid administration of antiserum is of primary importance. The sooner diphtheria antitoxin is given, the more favorable the outcome. Antitoxin is most effective if given within 4 days after the onset of illness. The antibody blocks entry of toxin into cells and therefore is effective only in neutralizing toxin in the extracellular space before entry into the cytoplasm. Dosage of antitoxin is adjusted to the severity of disease. Patients with extensive involvement of the tonsils and pharynx or larynx, who are expected to have higher concentrations of toxin, should be given at least half the treatment dose of antitoxin by intravenous infusion over 60 minutes. If extensive disease has been present for 3 or more days or if a bull neck has developed, administration of 80,000 to 120,000 units is recommended. For milder disease of shorter duration (48 hours or less), 20,000 to 40,000 units may be used. Antitoxin is given once; repeated doses provide no added benefit. Because antiserum is derived from horse serum, approximately 10% of patients have an allergic reaction. If skin or eye testing demonstrates hypersensitivity, de-sensitization should be attempted.
Antibiotic Therapy
Antibiotic therapy should be initiated as soon as possible and serves three purposes: (1) it shuts off toxin production; (2) it eradicates other potential pharyngeal pathogens, including group A streptococci; and (3) it eliminates the carrier state, preventing the spread of C. diphtheriae to other nonimmunized persons. Erythro-mycin is considered the treatment of choice. Penicillin is also effective; it is given intramuscularly until the patient is able to swallow, then orally (as penicillin V) for the remainder of the 2-week course [see Table 1]. A study of Vietnamese children found penicillin to be more effective than erythromycin.15 C. diphtheriae is generally susceptible to clindamycin and rifampin. However, there has been less experience with the use of these antimicrobial agents.
Other Measures
Extensive pharyngeal and laryngeal membrane formation can lead to upper airway obstruction. Therefore, patients need to be closely monitored, and if signs of obstruction are detected, prompt intubation or tracheostomy must be performed. Sedatives may obscure the development of respiratory difficulties and should be avoided. Patients with myocarditis need cardiac monitoring and should initially be kept at bed rest. Treatment of heart failure with digoxin may result in further impairment of electrical conduction and lead to heart block. Experience with cardiac pacemakers is limited, but pacemakers would be expected to reduce mortality from complete heart block.
Table 1 Antibiotic Treatment of Infections Caused by Gram-Positive Bacilli
|
Pathogen (Disease) |
Drug |
Dosage |
Comment |
|
Corynebacterium diphtheriae (diphtheria) |
Erythromycin |
500 mg I.V. or p.o., q.i.d. x 2 wk |
First choice |
|
Penicillin |
Penicillin G, 600,000 U I.M. b.i.d., then penicillin V p.o. 250 mg q.i.d. x 2 wk |
First choice; in children, penicillin may be more effective than erythromycin |
|
|
C. urealyticum |
Vancomycin |
1 g I.V. q. 12 hr x 2-3 wk |
Use antibiotic sensitivity testing to guide therapy |
|
Vancomycin |
1 g I.V. q. 12 hr x 2-3 wk |
First choice; use antibiotic sensitivity testing to guide therapy |
|
|
C. jeikeium |
Penicillin G + gentamicin |
20 million U/day I.V. divided q. 4-6 hr 5 mg/kg/day I.V. divided q. 8 hr |
Alternative |
|
C. ulcerans Rhodococcus equi |
Erythromycin |
500 mg I.V. or p.o., q.i.d. x 2 wk |
— |
|
Vancomycin ± rifampin ± erythromycin, imipenem, amikacin, or ciprofloxacin |
1 g I.V. q. 12 hr x 6 wk 600 mg p.o., q.d. 1 g I.V. q. 6 hr 0.5-1 g I.V. q. 6 hr 15 mg/kg I.V. q.d., or divided q. 8-12 hr |
Prolonged therapy often required, relapse common; combination therapy recommended; no one regimen has proved to be more effective |
|
|
500-750 mg p.o. or 400 mg I.V. q. 12 hr |
|||
|
Listeria monocytogenes |
Ampicillin ± gentamicin |
2 g I.V. q. 6 hr x 3-6 wk 5 mg I.V. q.d. or divided q. 8 hr |
Clinical efficacy of gentamicin has not been proved |
|
Trimethoprim-sulfamethoxazole (TMP-SMX) |
20 mg TMP, 100 mg SMX/kg/day I.V., divided q. 6-8 hr x 3-6 wk |
Use in penicillin-allergic patients |
|
|
Nocardia |
Sulfisoxazole or sulfadiazine |
1.5-2 g I.V. q. 6 hr |
May not be available on hospital formulary; follow by oral sulfonamide x 10-11 mo |
|
TMP-SMX |
20 mg TMP, 100 mg SMX/kg/day I.V., divided q. 6-8 hr x 1-2 mo |
Follow by oral sulfonamide x 10-11 mo |
|
|
Minocycline |
100 mg I.V. q. 12 hr |
Alternative drug; may cause vertigo |
|
|
Imipenem ± amikacin |
0.5-1 g I.V. q. 6 hr 15 mg/kg I.V. q.d. or divided q. 8-12 hr |
Alternative |
Isolation and Treatment of the Carrier State
When diphtheria is suspected, the patient should be isolated until two cultures from the infected site are negative. Cultures should be obtained from all persons who have been in close contact with the patient to determine whether they are pharyngeal carriers. All carriers need to be treated with erythromycin or penicillin for 14 days, and eradication of the carrier state must be documented by follow-up cultures.