Taxonomy
Like other gram-negative bacteria, Rickettsia and Ehrlichia are members of the alpha group of purple bacteria. In the past, the family Rickettsiaceae was divided into three tribes: Ehrlichiae, Rickettsiae, and Wolbachia. The tribe Ehrlichiae was divided into three genera: Ehrlichia, Cowdria, and Neorickettsia. A new taxonomy of existing organisms has been proposed [see Figure 1] that is based on genetic analysis and divides the order Rick-ettsiales into two families, Rickettsiaceae and Anaplasmataceae; however, this taxonomy is still controversial and is likely to change in the future. The family Rickettsiaceae contains the genera Orientia and Rickettsia. The genus Rickettsia is divided into the typhus and spotted-fever groups, which in turn contain numerous species. For example, over 15 different spotted-fever-group rickettsial species have been named. The family Anaplasma-taceae is divided into a complex number of Anaplasma and Ehrlichia genera. Some organisms previously considered to be Ehrlichia have been renamed Anaplasma.
Although the term ehrlichiosis is generally used by clinicians to describe tick-borne diseases caused by organisms in the genera Ehrlichia and Anaplasma, some organisms that were previously considered to be (and are still widely considered to be) Ehrlichia have been assigned to the genus Anaplasma. For example, the agent of human granulocytic ehrlichiosis (HGE) has been renamed A. phagocytophilum (some sources list this as A. phagocytophilia or A. phagocytophila). Although this topic uses the old term, HGE, some authors now call this disease human granulocytic anaplasmosis. Moreover, some authors now use the term anaplasmosis instead of ehrlichiosis to describe infection by agents in the family Anaplasmataceae. To make taxo-nomic matters even more complicated, a surprising number of new spotted-fever-group rickettsial species have been discovered over the past 10 years. Additionally, previously recognized spotted-fever-group Rickettsia species (e.g., R. parkeri) that were thought not to cause human disease were recently confirmed to be human pathogens.
Biology and Ecology
Rickettsia are gram-negative bacteria that are difficult to see in tissue without the use of special stains. Rickettsia can usually be visualized using Gimenez or special immunoperoxidase stains or can be detected in tissue by the use of direct fluorescent antibody staining techniques.
Rickettsia organisms cannot be propagated on cell-free media, but they can be grown in tissue cultures or in the yolk sacs of developing chick embryos. Members of the spotted-fever group, such as R. rickettsii, grow in both the nucleus and the cytoplasm of host cells, whereas typhus-group Rickettsia organisms grow only in the cytoplasm of infected host cells. Rickettsial cell walls contain lipopolysaccharides and outer-membrane proteins (rOmps). These rOmps are capable of eliciting a protective immune response in animals. Their exact function in arthropods and mammalian cells is uncertain.
Ehrlichia and Anaplasma organisms may infect monocytes (in human monocytic ehrlichiosis [HME]) or granulocytes (in HGE).
Figure 1 Taxonomy of the rickettsias.
Ehrlichia organisms replicate in the phagosomes of the host cell. Like Rickettsia organisms, Ehrlichia organisms have distinct ribo-somes and are surrounded by an outer and inner membrane. Ehrlichia organisms can be detected by light microscopy when they exist as single organisms and can be stained with Gram, Giemsa, Wright, or silver stains. Ehrlichia organisms cannot be grown on cell-free media and thus far have been cultured only in eukaryotic cells and in yolk sacs.
Most Rickettsia and Ehrlichia organisms cycle between arthropod vectors and small mammals, infecting humans only as an incidental host. A notable exception to this pattern is R. prowazekii, which is primarily maintained in a human-louse cycle.
Pathophysiology
After inoculation into humans, Rickettsia organisms proliferate intracellularly and then spread throughout the body via the bloodstream or lymphatics. Spotted-fever-group Rickettsia species, such as R. rickettsii, have a specific and characteristic tro-pism for endothelial cells. After attachment to the host endothe-lial cell plasma membrane via a cholesterol-containing receptor, these organisms enter the cell via pinocytosis and then escape from phagosomes into the cytosol. R. rickettsii divides by binary fission and then spreads cell to cell via filopodia derived from host cell membranes.
The hallmark of most rickettsial diseases is cell injury or death associated with an intense vasculitis. Widespread Rickettsia-in-duced vasculitis in humans leads to minute focal areas of hemorrhage and edema (as a result of increased vascular permeability), along with activation of the humoral immune response, inflammation, and coagulation. Vasculitis from rickettsial infection may result in hypotension, shock, widespread organ dysfunction, and death. Although the histopathologic and clinical features of rickettsial infection have been well characterized, the precise cellular mechanism by which most Rickettsia organisms produce cell damage to small blood vessels is unknown.
Individual Ehrlichia and Anaplasma species infect hosts ranging from humans to dogs, sheep, and horses. Four species are known to infect humans: E. chaffeensis (the organism responsible for human monocytic ehrlichiosis), A. phagocytophilum (the agent of HGE), E. sennetsu, and E. ewingii.
Ehrlichia organisms enter host cells by phagocytosis and then grow and divide while in the host cell phagosome. After a few days, tightly packed clusters of organisms are observable as in-tracellular inclusions. Later, additional growth and replication of Ehrlichia organisms result in large, mature inclusions called morulae. In vitro growth of Ehrlichia and Anaplasma in tissue culture or yolk cell systems takes 8 to 36 days. Only a few isolates of E. chaffeensis and A. phagocytophilum have been cultured from humans, because in vitro cultivation of these organisms from clinical specimens is extremely difficult.
There is no serologic cross-reactivity between Ehrlichia and Rickettsia, although some cross-reactivity exists between E. chaf-feensis, E. ewingii, and A. phagocytophilum. In animals, both experimentally induced and naturally acquired Ehrlichia infections may result in antibody responses, but these responses may not be protective. The relationship between humoral antibody responses and immunity in human ehrlichiosis is still not understood.
How Ehrlichia produces human disease is also poorly understood. Ehrlichia does not cause vasculitis, nor do human tissues infected with Ehrlichia demonstrate cell necrosis, abscess formation, or a severe inflammatory response. Patients infected with Ehrlichia may develop lymphadenopathy and perivascular lym-phohistiocytic infiltrates without apparent endothelial injury or thrombosis. Some patients with A. phagocytophilum infection contract secondary opportunistic fungal infections. There is also no understanding of the mechanism for the thrombocytopenia that occurs, predictably and early, in most forms of ehrlichiosis. Studies in animal models have shown that decreased hematopoietic production, immune-mediated platelet destruction, and splenic sequestration are unlikely to be the primary mechanism for this thrombocytopenia.2
Rocky Mountain Spotted Fever
Epidemiology
Rocky Mountain spotted fever (RMSF) is an acute tick-borne illness caused by R. rickettsii. RMSF occurs widely in the Western Hemisphere, from southern Canada to Mexico. RMSF also occurs in Central America and has been described in Colombia and Brazil. The primary vectors of RMSF are the Rocky Mountain wood tick in the western United States and the American dog tick in the eastern United States. Other tick vectors are responsible for transmission in Mexico, Central America, and South America. Ticks represent both the reservoir and the vector of RMSF. R. rickettsii normally cycles between ticks and small mammals such as moles and rodents; humans are only an incidental host.
Although RMSF occurs sporadically in most of the continental United States in rural, suburban, and urban locations, it is most often reported from the southeastern and south central United States. Cases of RMSF have been reported from Central Park in New York City and from suburban areas such as Long Island, New York. Over 90% of all cases of RMSF occur from early spring to early autumn.
Up to one third of patients with proven RMSF cannot recall a history of tick bite or recent tick contact. Rickettsial transmission in such cases presumably occurs through inapparent or unrecognized tick bite or through contact with infected tick tissues.
Diagnosis
Clinical Manifestations
RMSF typically begins abruptly. The incubation period after tick bite or tick exposure ranges from 2 to 12 days. The early phases of illness are usually marked by nonspecific symptoms such as fever, headache, malaise and myalgias, and nausea and vomiting. Some patients, especially children, have prominent abdominal pain; this pain may be severe enough to mimic an acute surgical abdomen.
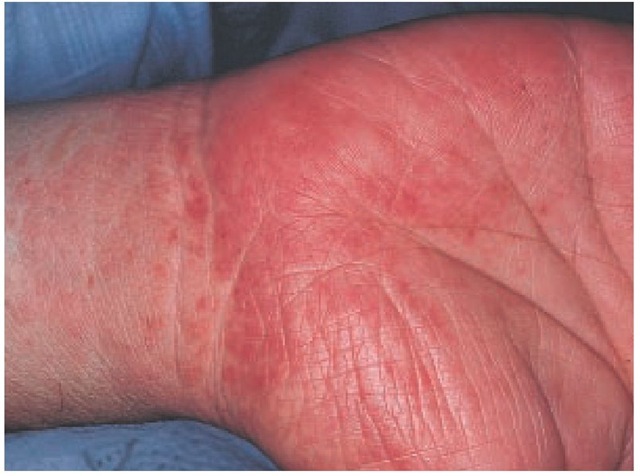
The skin rash that gives RMSF its name may begin as a macu-lar or maculopapular eruption and evolve into a generalized pe-techial rash [see Figure 2]. Generalized ecchymosis and even gangrene of the digits, genitals, ears, and nose may rarely occur in severe cases.3 The rash typically develops on the third to the fifth day of illness. Appearance of the rash may be delayed, however; and in a small percentage of patients, the rash does not develop at all. Delay or absence of the rash greatly complicates clinical diagnosis. In one study, only 14% of RMSF patients had a rash on the first day of illness and fewer than 50% developed a rash in the first 72 hours of illness.4 Absence of rash does not correspond to milder disease; a small percentage of patients with so-called spotless RMSF have fatal illness.5 Moreover, the characteristic skin rash of RMSF may be overlooked in dark-skinned patients.
Figure 2 Purpuric macules on the palm and wrist of a patient with Rocky Mountain spotted fever.
Patients with RMSF may occasionally present with a wide variety of neurologic symptoms ranging from confusion to seizures and encephalopathy. Some patients with RMSF have focal neurologic signs that lead to misdiagnoses. Neurologic symptoms may persist after recovery of acute illness in some patients.6 Other atypical and uncommon presenting features of RMSF include periorbital edema (especially in children) and cough.
The diagnosis of RMSF is notoriously difficult, even for experienced physicians in highly endemic areas. In the first few days of illness, particularly when the characteristic rash is absent, RMSF may mimic an array of nonspecific viral diseases. If early RMSF is mistaken for a bacterial illness and if ^-lactams or other antibiotics ineffective against R. rickettsii are given, the subsequent appearance of a rash may be tragically mistaken for a drug eruption and lifesaving therapy may be omitted or delayed. RMSF has been confused with measles, staphylococcal bac-teremia, hepatitis, leptospirosis, meningococcemia, and infectious mononucleosis.7 The clinical features of RMSF and ehrlichiosis are often similar [see Table 1]. Even experienced physicians cannot always distinguish the two diseases, although the presence of severe leukopenia and the absence of rash favor the diagnosis of ehrlichiosis.8
It is axiomatic that the diagnosis of RMSF must be based on the clinical features and an appropriate epidemiologic setting rather than on any single laboratory test. There is no completely reliable diagnostic test for RMSF in the early phases of illness; thus, therapy should always begin before laboratory confirmation is obtained.
Laboratory Studies
The white blood cell count remains normal in most patients with RMSF. In some cases, however, the WBC may be low or elevated. Thrombocytopenia generally occurs in severe cases and is a helpful diagnostic finding. The low platelet count may be accompanied by reduced fibrinogen concentrations and elevated levels of fibrin split products. Other characteristic laboratory abnormalities in patients with RMSF include elevated blood levels of aminotransferases, bilirubin, and creatinine.
Diagnostic proof of RMSF can be obtained by direct immuno-fluorescent or immunoenzyme staining of skin biopsy samples or, in the convalescent phase of the disease, by the detection of characteristic antibodies. These antibodies typically do not appear before day 8 to 10 of illness; thus, serologic testing has no role in the initial diagnosis of acutely ill patients with suspected RMSF.
Culture of Rickettsia from blood samples can be done only in specialized laboratories and the delay in obtaining a result makes positive cultures useful only in retrospect. Polymerase chain reaction technology has been applied to the diagnosis of RMSF, but such testing is neither sensitive nor widely available. Because some patients die before generating an antibody increase against R. rickettsii antigen, the diagnosis in fatal cases may be completely missed unless immunohistochemical staining is performed on tissues obtained at autopsy.9
Treatment
Tetracyclines and chloramphenicol are the only antibiotics known to be effective against R. rickettsii. Doxycycline is the preferred agent in all patients except pregnant women, for whom chloramphenicol remains the agent of choice. Therapy should be initiated as early as possible in the course of RMSF. Therapy started more than 5 days after onset is much less likely to be effective than therapy given earlier.
The usual dosage of doxycycline is 200 mg a day in two divided doses. The same dosage can be administered intravenously or orally. Intravenous therapy is preferred for patients who are seriously ill or who are experiencing nausea or vomiting. Children with RMSF should be given doxycycline at a dosage of 2.5 to 3 mg/kg/day in two divided doses. Chloramphenicol given at an initial loading dose of 50 mg/kg, followed by 50 mg/kg/day in four divided doses, is an appropriate alternative for patients who cannot tolerate tetracyclines or who are pregnant [see Table 2].
Unfounded fears about the risk of tetracycline therapy in children with suspected rickettsial disease may unnecessarily delay the institution of potentially lifesaving therapy.11 Because RMSF can normally be cured with a short course of doxycycline therapy, concerns about staining of the teeth should not prohibit the use of doxycycline in young children with suspected RMSF; indeed, such therapy may be lifesaving, and it is considerably safer than therapy with chloramphenicol. In addition to antimicrobial therapy, treatment should include measures to correct associated complications, such as hypotension, heart failure, and electrolyte disturbances. Glucocorticoid therapy has never been shown in a controlled study to be useful in the treatment of RMSF, and it is not recommended.
Prevention
The prevention of RMSF relies on avoidance of tick-infested areas or the use of protective clothing and tick repellent while in such areas. Prompt removal of ticks after attachment is particularly important. Ticks should be removed using tweezers, tissue, or cloth to protect the fingers, because crushed tick tissues can be infectious.
Prognosis
In consequence of the difficulty in making a conclusive diagnosis of RMSF in the early phases of illness, delays in the initiation of therapy are common. Such delays are often associated with fatal outcome if they exceed 5 days. A number of host factors have been associated with severe or fatal RMSF, including increasing age, male gender, and the presence of glucose-6-phospate dehydrogenase (G6PD) deficiency. Black race and alcohol use have also been associated with more severe disease and a higher fatality, but it is difficult to exclude the role of delay in seeking or receiving antimicrobial therapy in these patients. The biologic causes for the worse outcomes seen in patients older than 40 years and in male patients remain poorly understood. Patients with abnormal renal function at the time of presentation have a worse prognosis than those with normal renal function.
Table 1 Diagnosis of Diseases Caused by Rickettsia, Ehrlichia, and Coxiella Species
PCR—polymerase chain reaction
TIBOLA—tick-bite-associated lymphadenopathy
Rickettsialpox
Rickettsialpox is an uncommon mite-borne rickettsial disease caused by R. akari. The disease’s name is derived from its clinical resemblance to chickenpox.