Cyclooxygenase-1 and Cyclooxygenase-2
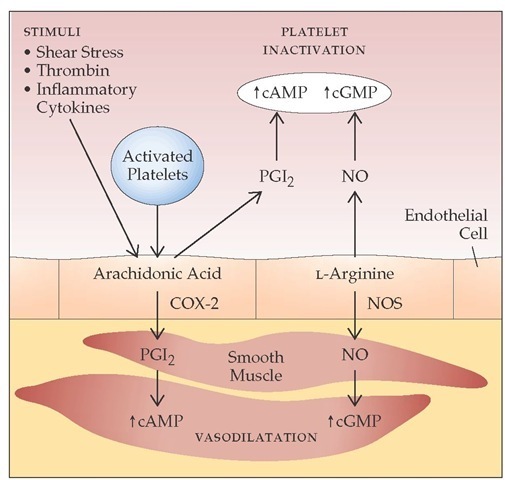
Cyclooxygenase-1 (COX-1) is the constitutive isoform of PGHS. Cyclooxygenase-2 (COX-2) is an inducible isoform of PGHS. COX-2 is undetectable in most tissues. However, it can be rapidly induced in response to growth factors, endotoxins, and cy-tokines in endothelial cells and monocytes (although not in platelets).14 Recent evidence indicates that endothelial COX-2 is a major source of PGI2 under physiologic conditions in humans, perhaps because of continual COX-2 induction by hemodynam-ic shear in the circulation.15 Aspirin acetylates and irreversibly inhibits both COX-1 and COX-2. Other nonsteroidal anti-inflammatory drugs (NSAIDs) also inhibit COX-1 and COX-2, although not permanently. Selective COX-2 inhibitors are now available as a new generation of NSAIDs.16
Because aspirin irreversibly inhibits COX-1 and because platelets cannot make new COX-1, brief exposure to aspirin will permanently inhibit TXA2 production for the life span of affected platelets.
Nitric oxide
Nitric oxide (NO) is formed from L-arginine in endothelial cells. NO stimulates guanylate cyclase, leading to an increase in cyclic guanosine monophosphate (cGMP) in target cells; causes vasodilatation; and inhibits platelet adhesion and aggregation [see Figure 8].17 NO is rapidly destroyed by hemoglobin and thus functions as a local (i.e., paracrine) hormone. Intravenous infusion of an arginine analogue that blocks NO production leads to an immediate and substantial rise in blood pressure. This phenomenon suggests that NO is released continually and basally to regulate vascular tone (in contrast to the production of PGI2, which is more stimulus-responsive). There is significant syner-gism between NO and PGI2. Formation of NO is catalyzed by NO synthases, which exist in different isoforms in various tissues. In addition to regulating vascular events, NO has a wide range of biologic effects (e.g., neurotransmittal function in the central nervous system).
ECTO-ADPase (CD39)
CD39 is an integral membrane protein found on the endothe-lial cell surface. It is an active enzyme that rapidly hydrolyzes ADP to AMP, thus functioning as a cell-bound ecto-ADPase. It limits the recruitment of additional platelets into the growing platelet plug by removing ADP released from the dense granules of activated platelets and from damaged erythrocytes and endothelial cells.
Figure 8 Significant synergism exists between nitric oxide (NO) and prostacyclin (PGI2), leading to platelet inactivation and vasodilatation. The enzyme prostaglandin endoperoxide H synthase-1 (PGHS-1) converts arachidonic acid into PGI2 in endothelial cells. PGI2 activates adenylate cyclase, which leads to an increase in intracellular cyclic adenosine monophosphate (cAMP), inhibiting platelet aggregation and inducing vasodilatation. NO, formed from L-arginine, stimulates production of cyclic guanosine monophosphate (cGMP). Cyclooxygenase-2 (COX-2) is the induced isoform of PGHS; its formation presumably results from hemodynamic shear in the circulation. NO formation is catalyzed by NO synthases (NOS).
Fibrinolysis
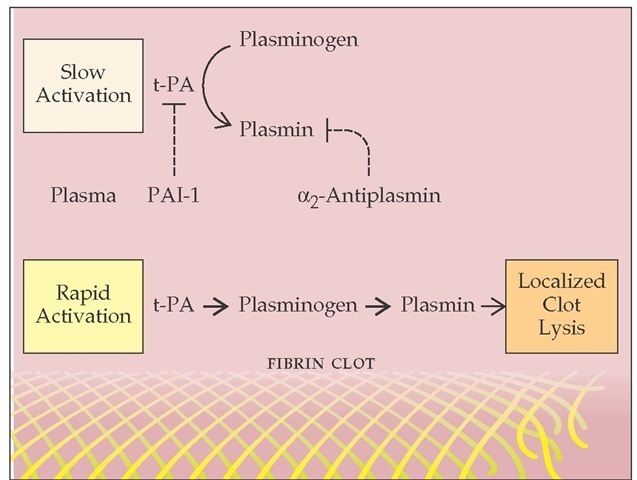
Tissue plasminogen activator (t-PA) is released from perturbed endothelial cells near the site of vascular injury. t-PA converts plasminogen to plasmin. Like the AT-III interaction with thrombin, which is accelerated in the presence of endothelial cell surface heparan sulfate, generation of plasmin takes place optimally on a surface (in this case, the fibrin clot). Both t-PA and plasminogen bind to fibrin (via recognition of lysine residues), which facilitates plasmin generation and localized fibrinolysis [see Figure 9].
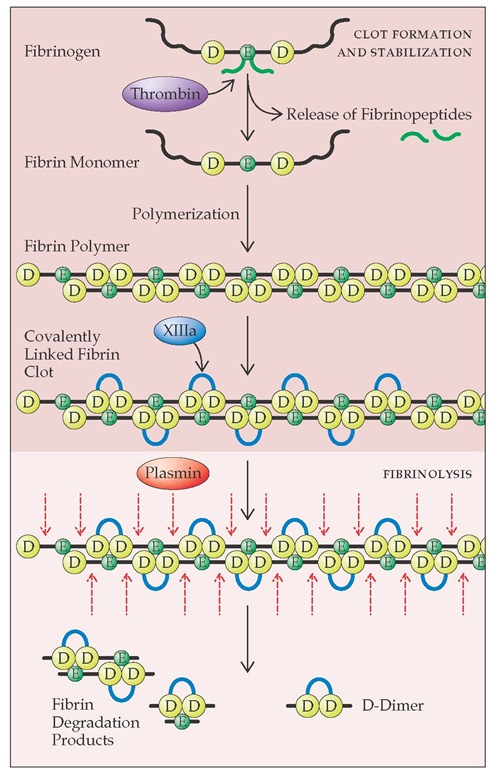
Plasmin cleaves the polymerized fibrin strand at multiple sites, releasing fibrin degradation products. One of the major fibrin degradation products is D-dimer, which consists of two D domains from adjacent fibrin monomers that have been cross-linked by activated factor XIII [see Figure 10]. Plasmin has a broad substrate specificity and, in addition to fibrin, cleaves fibrinogen and a variety of plasma proteins and clotting factors. Plasmin bound on the fibrin clot is protected from inactivation, whereas plasmin released into the circulation is rapidly inactivated by plasma a2-antiplasmin. Thus, localized fibrinolysis is achieved, but nonspecific plasmin degradation of plasma proteins is prevented. In rare cases, patients have bleeding problems caused by a congenital deficiency in a2-antiplasmin.
Urokinase is the second physiologic plasminogen activator. It is present in high concentration in the urine. Although t-PA is largely responsible for initiating intravascular fibrinolysis, uroki-nase is the major activator of fibrinolysis in the extravascular compartment. Urokinase is secreted by many cell types in the form of prourokinase, also termed single-chain urokinase-type plasminogen activator (scu-PA). Prourokinase is converted to urokinase by plasmin. Urokinase lacks fibrin specificity in converting plasminogen to plasmin, whereas prourokinase displays such specificity.
The major physiologic inhibitor of t-PA and urokinase plas-minogen activator (u-PA) is plasminogen activator inhibitor-1 (PAI-1).19 Substantial amounts of PAI-1 are found in platelets. PAI-1 is also released from endothelial cells. PAI-1 deficiency is associated with bleeding diathesis, usually related to trauma or surgery.20 A second inhibitor, PAI-2, is normally secreted by monocytes. During pregnancy, PAI-2 levels are greatly increased because of synthesis by the placenta. The biologic importance of PAI-2 remains to be established.
Thrombin-Activatable Fibrinolysis Inhibitor
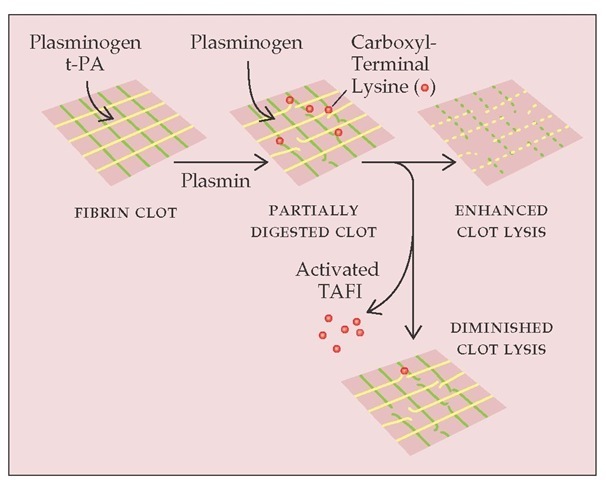
Plasma carboxypeptidase is a newly recognized thrombin-ac-tivatable fibrinolysis inhibitor (TAFI) [see Figure 11].21,22 TAFI is the second known physiologic substrate for the thrombin-thrombomodulin complex. One may envisage that after the initial fibrin clot is formed by thrombin at the site of a vascular wound, thrombin binds to thrombomodulin on the nearby intact endothelial surface. The thrombomodulin-bound thrombin leads to the generation of activated protein C, which dampens the clotting cascade and prevents excessive thrombin generation. At the same time, the thrombomodulin-bound thrombin activates TAFI, thus slowing down the lysis of the existing clot. In hemophilia, the decreased generation of thrombin may lead to suboptimal activation of TAFI and result in premature clot lysis, which contributes to the delayed bleeding observed in these pa-tients.22 Whether excessive TAFI activity leads to thrombosis is unknown at present.
Overview of Blood Coagulation
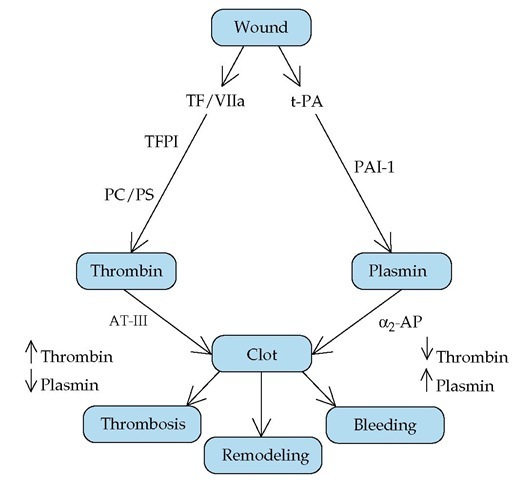
The clotting cascade is initiated by the exposure of tissue factor at a vascular wound, which leads to the generation of thrombin and the deposition of a fibrin clot [see Figure 12]. Simultaneously, the damaged endothelium releases t-PA, which converts plasminogen to plasmin, which then lyses the clot.
Figure 9 Tissue-type plasminogen activator (t-PA), released from perturbed endothelial cells near an injured blood vessel, converts plasminogen to plasmin. Free plasmin is rapidly inactivated by plasma a2-antiplasmin; plasmin bound to the fibrin clot is protected from inactivation.
Figure 10 The transformation of fibrinogen to fibrin is initiated by thrombin cleavage of fibrinopeptides A and B from the E domains of fibrinogen to form fibrin monomer. The cleavage apparently changes the overall negative charge of the E domain to a positive charge. This change in charge permits the spontaneous polymerization of fibrin monomers, because the positively charged E domain assembles with the negatively charged D domains of other monomers. The polymer is initially joined by hydrogen bonds. Thrombin activates factor XIII, which catalyzes the formation of covalent bonds between adjacent D domains in the fibrin polymer. Plasmin cleaves the polymerized fibrin strand at multiple sites and releases fibrin degradation products, including D-dimer.
Both pathways are regulated: TF/factor VIIa is regulated by the TFPI/factor Xa complex, and thrombin is regulated by protein C and protein S. Similarly, the activity of t-PA is regulated by PAI-1. Thrombin and plasmin are under the control of their respective inhibitors, AT-III and a2-antiplasmin. When these two pathways work in coordinated symmetry, a clot is laid down to stop bleeding, and clot lysis and tissue remodeling follow. Diminished thrombin generation (as in factor VIII deficiency) or enhanced plasmin production (as in a2-antiplasmin deficiency) causes hemorrhage [see 5:XIII Hemorrhagic Disorders]. Conversely, excessive production of thrombin (as in AT-III or protein C deficiency) leads to thrombosis [see 5:XIV Thrombotic Disorders].
Heterogeneity of Endothelial Cells and Vascular Bed-Specific Hemostasis
Although the endothelium is generally considered to be a distinct, homogeneous organ system, there are significant differences between arterial, venous, and capillary endothelial cells in terms of morphology and disease susceptibility. Recent studies have shown distinct sets of proteins that mark the arterial and venous endothelial cells from the earliest stages of angiogenesis. Ephrin-B2, an Eph family transmembrane ligand, marks arterial but not venous endothelial cells. Conversely, Eph-B4, a receptor tyrosine kinase for ephrin-B2, marks veins but not arteries.23
It is also likely that endothelia from different vascular beds are not identical.24 For example, the high endothelium in the post-capillary venules of lymph nodes and Peyer patches regulates the circulation of lymphocytes from blood to lymphatics and peripheral tissues. Specific adhesive protein receptors and matrix proteins are highly expressed in these high endothelial venules. The specialized endothelium representing the blood-brain barrier is another example.
These differences between arterial and venous endothelial cells and the vascular bed-specific endothelium may partly account for their different susceptibilities to thrombosis. For example, whereas AT-III and protein C deficiencies are usually associated with deep vein thrombosis of the lower extremities, thrombosis of portal and hepatic veins is frequently associated with myeloproliferative diseases.25 In both conditions, the underlying defect is a systemic hypercoagulable state, and yet there is a clear predisposition of thrombosis to specific vascular beds. Thus, clinical thrombosis is attributable to an imbalance between systemic prothrombotic stimuli and local antithrombotic mechanisms [see 5:XIV Thrombotic Disorders].
Platelet Production and Thrombopoietin
Platelets are derived from megakaryocytes, which arise from pluripotent myeloid stem cells. Platelet production is controlled by a thrombopoietin that is involved in the final maturation of the megakaryocyte. Thrombopoietin has multiple actions in megakaryocyte development.26 It shares some structural homol-ogy with erythropoietin and is produced principally by the liver. It increases the size and number of megakaryocytes, stimulates the expression of platelet-specific markers, and is a potent megakaryocyte colony-stimulating factor. Although throm-bopoietin is clearly a key factor, stem cell factor (also called kit ligand), interleukin-3 (IL-3), IL-6, and IL-11 all play contributory roles in controlling megakaryocytopoiesis.
Figure 11 Plasma carboxypeptidase is a thrombin-activatable fibrinolysis inhibitor (TAFI). When fibrin is degraded by plasmin, new carboxyl-terminal lysines are exposed in the partially digested clot. These lysines provide additional sites for plasminogen incorporation and activation in the clot, setting up a positive feedback loop in clot lysis. Thrombin activates carboxypeptidase-B in plasma, which removes the exposed carboxyl-terminal lysines and prevents further plasminogen incorporation into the clot.
Figure 12 Exposure of tissue factor at a vascular wound initiates the clotting cascade. Generation of thrombin and deposition of a fibrin clot occur simultaneously with release of t-PA from the damaged epithelium and conversion of plasminogen to plasmin. Plasmin then lyses the clot. When these two pathways work in coordinated symmetry, a clot is laid down to stop bleeding, and clot lysis and remodeling follow. (a2-AP—a2-antiplasmin; AT-III—antithrombin III; PAI-1—plasminogen activator inhibitor-1; PC/PS—protein C/protein S; TF—tissue factor; TFPI—tissue factor pathway inhibitor; t-PA—tissue-type plasminogen activator)
Megakaryocytes undergo endomitosis, in which nuclear divisions occur without cell division and are followed by nuclear fusion, to yield a cell with a chromosomal content of 8n, 16n, or 32n. The megakaryocyte cytoplasm then changes into a series of thin, cylindrical strands that eventually fragment into small pieces of megakaryocytes, called proplatelets, that are released into the circulation. Megakaryocyte volume correlates with ploidy and cytoplasmic maturity; the largest megakaryocytes produce the greatest number of platelets. Large platelets called megathrombocytes are seen in the peripheral blood in thrombo-cytopenic states, especially in idiopathic thrombocytopenic pur-pura [see 5:XIIIHemorrhagic Disorders]. These megathrombocytes probably are young proplatelets and account for the increase in mean platelet volume that occurs during response to or recovery from acute thrombocytopenia.
Platelets entering the circulation survive about 8.5 to 10 days and have a half-life of about 4 days. Approximately 30% to 40% of the platelets are present in a splenic pool that can freely exchange with the circulation. When the need for platelets arises,production can increase sevenfold to eightfold. Because there is no marrow pool of platelets waiting to be released, meeting increased requirements for platelets may require a few days. Platelets have receptors for thrombopoietin and remove it from plasma, and the platelet mass functions as a major thrombopoi-etin regulator.27 In states of megakaryocyte hypoplasia and thrombocytopenia, little thrombopoietin is metabolized and the plasma thrombopoietin level rises, leading to increased production of megakaryocytes and platelets. In the setting of thrombocytosis, thrombopoietin metabolism increases, lowering the plasma thrombopoietin level and decreasing platelet production.