Anti-Glomerular Basement Membrane Disease
Epidemiology Anti-GBM disease is rare, with an estimated annual incidence of 0.5 to 0.9 cases per million population in whites and an even lower incidence in other races. Most patients are male and in either the second to third or the sixth to seventh decades of life, but the disease has been reported in both men and women and at all ages. Exposure to pulmonary toxins, such as hydrocarbons, increases the incidence of anti-GBM disease.
Pathogenesis and histology Anti-GBM disease is caused by a nephritogenic antibody directed against a specific epitope on the noncollagenous (NC1) domain of the a3 chain of type IV collagen in the GBM.9,45 Type IV collagen also occurs in the alveolar basement membrane, so these antibodies may also result in pulmonary involvement. Anti-GBM disease is the term used if the disorder is restricted to the kidney (primary renal disease); Goodpasture syndrome refers to the constellation of glomeru-lonephritis, pulmonary hemorrhage, and anti-GBM antibodies46 [see 10:VII Vascular Diseases of the Kidney].
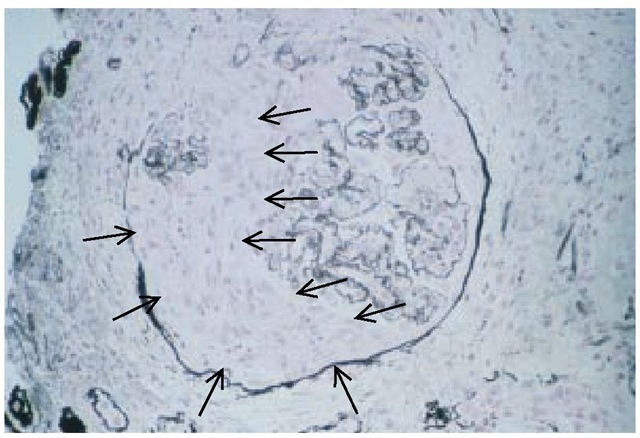
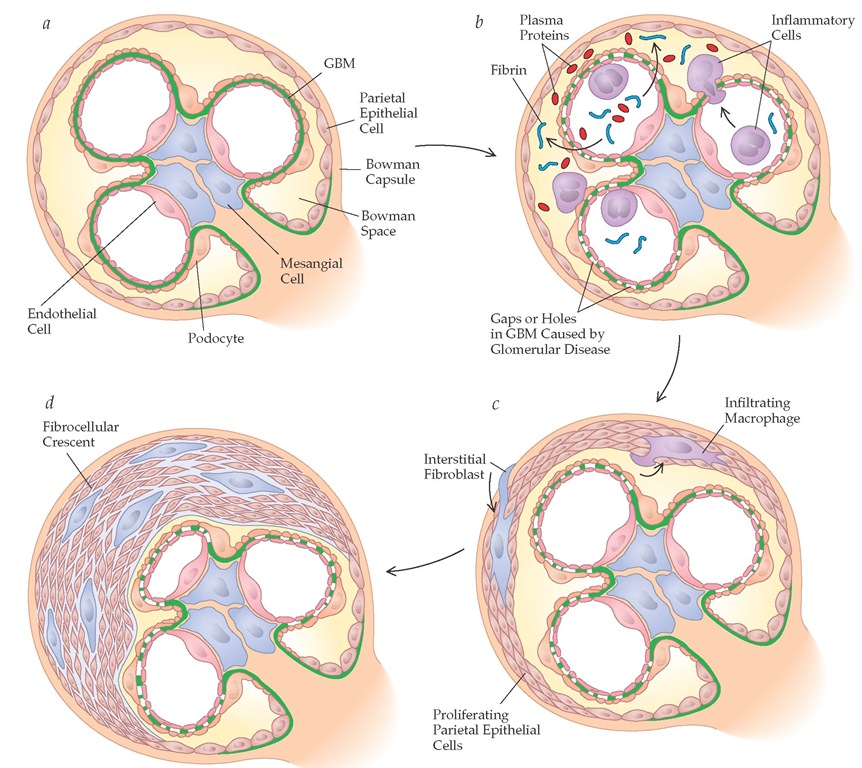
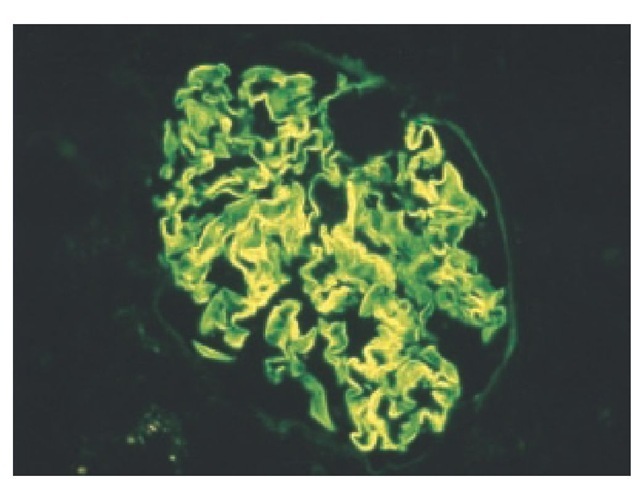
The characteristic finding on renal biopsy is a diffuse necrotiz-ing proliferative lesion (with increased cellularity because of proliferation) and crescents (layers of cells in the Bowman space comprising parietal epithelial cells, podocytes, macrophages, and leukocytes) in more than 50% of glomeruli [see Figure 7]. Although the initial injury occurs within the glomerular tuft, breaks in the GBM allow plasma products to leak from the capillaries into the Bowman space [see Figure #]. The early cellular and later fibrocellular crescents compress the glomerular tuft [see Fig-ure 8], so disease severity is related in part to the extent of crescents. Pathognomonic findings on renal biopsy in both anti-GBM disease and Goodpasture syndrome are linear deposition of IgG and C3 along the GBM [see Figure 9]. These findings are in contrast with the granular pattern of IgG immunostaining in immune complex glomerulonephritis (i.e., PSGN, IgA nephropa-thy, MPGN, and lupus nephritis).
Figure 7 A large multilayer of cells in the Bowman space (arrows) represents a typical crescent in anti-glomerular basement membrane disease.
Clinical and laboratory findings The typical presentation of anti-GBM disease consists of a rapid decline in renal function in a patient with a so-called active urinary sediment (i.e., containing RBCs and RBC casts and, occasionally, white blood cell [WBC] casts) and mild proteinuria, with or without the complications of pulmonary hemorrhage. There is a direct correlation between the initial plasma creatinine concentration and the percentage of glomeruli with crescents; crescents are present in more than 75% of glomeruli when plasma creatinine concentration is above 5 mg/dl.
Patients with pulmonary-renal syndrome (exemplified by Goodpasture syndrome) present with pulmonary hemorrhage, acute renal failure, and anemia; these patients may have a history of viral infection or exposure to pulmonary toxins such as cigarette smoke or hydrocarbons.47 Blood loss from pulmonary hemorrhage often leads to iron deficiency anemia.
The diagnosis is confirmed by an increase in serum anti-GBM antibody titers. Serum complement levels are normal, and ANCA assays are positive in 20% to 30% of patients. Despite the presence of ANCAs in some patients, anti-GBM disease is not classified as a renal vasculitis.
Treatment and prognosis Patients with anti-GBM disease require urgent therapy to prevent a progressive decline in renal function.
Figure 8 Schematic view illustrating the events leading to crescent formation. (a) Normal glomerulus. (b) Glomerular disease leads to gaps or holes in the glomerular basement membrane (GBM), resulting in (c) proliferation of parietal epithelial cells and ultimately (d) fibrocellular crescent formation.
Figure 9 Linear pattern of IgG immunostaining along the GBM in a patient with anti-GBM disease.
The treatment of choice is triple therapy, consisting of plasmapheresis plus prednisone and cyclophosphamide. The rationale is that plasmapheresis removes circulating anti-GBM antibodies and other mediators of inflammation, whereas pred-nisone and cyclophosphamide minimize new antibody formation. Pulse steroids (methylprednisone, 30 mg/kg or 1,000 mg I.V.) are given for 3 days, followed by high-dose oral prednisone (1 mg/kg/day) given for several months, with plasma exchanges (4 L/day) given daily or on alternate days until the anti-GBM antibody is undetectable.48,49 Cyclophosphamide is given orally at a dosage of 2 mg/kg/day. Therapy is generally continued for 9 to 12 months.
More than 70% of treated patients with anti-GBM disease recover renal function. Oliguria and a serum creatinine level greater than 6 mg/dl are poor prognostic findings; patients with this high a creatinine level are generally considered not to be candidates for triple therapy. Renal transplantation should be avoided until the anti-GBM antibody has been undetectable for 12 months.
Renal and Systemic Vasculitis
The kidney is often involved in systemic vasculitis; examples include microscopic polyangiitis, Wegener granulomatosis, Churg-Strauss syndrome, and isolated pauci-immune crescentic glomerulonephritis. These diseases are discussed in detail elsewhere [see 10:VII Vascular Diseases of the Kidney and 15:VIII Systemic Vasculitis Syndromes].
Rapidly Progressive Glomerulonephritis
RPGN, an acute nephritic syndrome, is distinguished from other forms of nephritic syndrome by its clinical presentation, glomerular histology, and etiology [see Figure 10].50 RPGN is diagnosed clinically as a doubling in serum creatinine level over 3 months because of an underlying glomerulonephritis.50 Frequently, renal function deteriorates over as little as days or weeks. Most authorities also require the presence of glomerular crescents on renal biopsy to confirm the diagnosis. Typically, if more than 50% of glomeruli contain crescents, renal function decreases acutely (i.e., over days to weeks). RPGN constitutes a medical emergency.
RPGN can occur as a primary renal disorder or as a consequence of systemic disease. Conventionally, RPGN is classified according to the underlying disease mechanism into one of three categories: 50% of cases are from pauci-immune disease, which is often associated with ANCAs; 30% are from immune complex disease (e.g., postinfectious glomerulonephritis, lupus nephritis, IgA nephropathy, and MPGN); and 20% of cases are from anti-GBM.51
Diseases presenting as nephrotic syndrome
Nephrotic syndrome is characterized by heavy proteinuria (> 3.5 g/24 hr), hypoalbuminemia (< 3 g/dl), peripheral edema, hyperlipidemia (elevated total and LDL cholesterol levels) and lipiduria (oval fat bodies in urinary sediment). Nephrotic syndrome results from a marked increase in glomerular permeability to protein and other macromolecules. Although glomerular injury is often severe in diseases causing nephrotic syndrome, it is unusual for patients to present with a decrease in renal function early in the course of disease. A rise in the serum creatinine level is usually a feature of more advanced disease. The urinary sediment is typically devoid of red and white cells or casts and is termed an inactive urinary sediment. This contrasts with the active urinary sediment in nephritic syndrome. However, some patients who present with a combined nephritic-nephrotic picture will have an active urinary sediment. In nephrotic patients with a marked increase in serum lipid levels, fat and cholesterol casts can be seen in the urine because of markedly increased glomerular permeability.
The hallmark of the nephrotic syndrome is proteinuria greater than 3.5 g/24 hr. It is very unusual for proteinuria of this magnitude to be caused by conditions other than glomerular disease. In the absence of a 24-hour urine collection, a spot urine collection can be used to estimate the degree of proteinuria.52 A normal urinary protein-to-creatinine ratio (mg/mg) is less than 0.15, which indicates a protein excretion of less than 150 mg/day; a ratio greater than 3.5 is consistent with excretion of more than 3.5 g of protein, which defines nephrotic syndrome. A urine dipstick detects only albumin, not other proteins, and is positive only when protein excretion exceeds 300 to 500 mg/day. Caution must also be used when interpreting dipstick units, however, because urine tonicity or specific gravity affects the measurement. In certain circumstances (e.g., Bence Jones proteinuria in multiple myeloma), the dipstick will be negative for protein.
Lesser degrees of proteinuria (< 3.5 g/24 hr) may result from glomerular or nonglomerular causes. These include heart failure; renal vascular disease; increased GFR from exercise, pregnancy, diabetes, or fever; tubulointerstitial disorders; increased protein delivery from myeloma; and orthostatic proteinuria (a benign condition in which low-grade proteinuria occurs only when the patient is not recumbent).
Although nephrotic syndrome is caused by an underlying glomerular disease, progressive renal failure is caused by tubu-lointerstitial inflammation and scarring that results from ongoing proteinuria. Thus, the renal biopsy must also be evaluated for the presence of tubulointerstitial fibrosis.
The etiology of nephrotic syndrome is separated into primary and secondary causes. The most common primary glomerular diseases that cause nephrotic syndrome in adults are FSGS, membranous nephropathy, minimal change disease, and MPGN [see Table 1]. Although these disease entities are usually primary (and idiopathic), each can also be secondary to an underlying systemic disease. However, the glomerular histology is indistinguishable in primary and secondary forms. Diabetes mellitus and amyloi-dosis are the leading causes of secondary nephrotic syndrome.
The target of injury in FSGS, membranous nephropathy, minimal change disease, and even diabetes is the podocyte [see Figure 1b]. A critical function of the podocyte, which sits on the outer aspect of the GBM, is to prevent proteinuria. Thus, the characteristic clinical consequence of podocyte damage is massive proteinuria. Moreover, because podocytes are located on the outer aspect of the GBM and therefore not in close proximity to the capillary loops, podocyte injury in nephrotic syndrome is typically not associated with red or white cells or cell casts in the urine.
Therapeutic Principles in Nephrotic Syndrome
Four principles guide the management of the nephrotic syndrome. First, treat any complications: reduce fluid overload with diuretics, provide anticoagulation therapy for patients at high risk for venous thrombosis (e.g., those with a serum albumin level < 2.0 g), aggressively treat hypertension (blood pressure goal < 130/80 mm Hg), and decrease hyperlipidemia with statins. Second, lower proteinuria to less than 1 g/24 hr, ideally through combination therapy with ACE inhibitors and ARBs. Third, use disease-specific therapy when possible. Fourth, treat the underlying secondary cause if one is present.
Complications of Nephrotic Syndrome
Major complications of nephrotic syndrome include hypoal-buminemia, edema, hypercholesterolemia, and hypercoagula-bility. Although the liver is capable of synthesizing large amounts of albumin, proteinuria is associated with increased tubular uptake of protein. In the tubules, protein is catabolized rather than recycled for further use.53 As a result, serum albumin levels decrease. Hypoalbuminemia decreases the plasma oncotic pressure, resulting in a decrease in effective circulating volume. This is followed by an increased proximal tubular sodium resorption because of increased angiotensin II, aldosterone, and epineph-rine. A concomitant increase in antidiuretic hormone levels results in fluid retention, which manifests clinically as edema and hypertension. Hypoalbuminemic nephrotic patients are often resistant to loop diuretics such as furosemide, because loop diuretics are inactivated by binding to albumin in the tubular lumen. Edema also appears to result from a primary defect in sodium excretion that leads to an expanded plasma volume, followed by transudation of fluid.
Hepatic cholesterol and lipid synthesis are increased in nephrotic patients, probably in response to decreased oncotic pressure.54 Moreover, there is decreased catabolism, which explains in part the increase in levels of very low density lipopro-tein. Hyperlipidemia is a major risk factor for cardiovascular disease in nephrotic patients. It may also contribute to the progression of glomerular diseases. Treatment of hyperlipidemia should be aggressive and includes cholesterol-lowering agents such as statin drugs [see 9:II Diagnosis and Treatment of Dyslipidemia].
Patients with nephrotic syndrome are in a hypercoagulable state: 10% to 40% will experience arterial or venous thromboem-bolism.55 Factors that underlie increased coagulation include urinary losses of the anticoagulant antithrombin, increased platelet activation, and alterations in the fibrinolytic system. Patients may present with lower extremity, pulmonary, or renal vein thrombosis. Renal vein thrombosis complicates all forms of the nephrotic syndrome—especially membranous nephropathy— and may present acutely as a sudden decrease in renal function, loin pain, hematuria, or even systemic emboli. However, thrombosis of the renal vein may also be asymptomatic, especially if collateral veins have been established. Diagnosis is made by renal vein Doppler sonography, CT angiography, or MR angiogra-phy. Treatment includes anticoagulation therapy with heparin followed by warfarin. Many authorities use prophylactic low-dose warfarin when the plasma albumin concentration is less than 2 g/dl [see 5:XV Thrombotic Disorders].
Other complications of the nephrotic syndrome include an increased susceptibility to infection (caused by urinary losses of IgG), bone disease (caused by loss of vitamin D), and loss of trace elements.