Clinical Classification of Glomerular Disease
A number of inflammatory and noninflammatory diseases can affect glomerular integrity and function. In general, nephrol-ogists use the term glomerulonephritis to denote glomerular diseases that result from an inflammatory process; these diseases also usually have an immunologic component. Important examples of glomerular diseases that are not caused by an inflammatory process include diabetic nephropathy [see 9:VI Diabetes Mellitus], hypertensive nephrosclerosis [see 1:III Hypertension], amyloidosis, and hereditary nephropathies (e.g., Alport syndrome). Diabetic nephropathy and hypertensive nephrosclerosis are the most common causes of chronic renal failure and end-stage renal disease in North America.
The clinical approach to glomerular disease often appears more complicated than it perhaps should be. This stems in part from the variety of classification systems used for glomerular disease, which categorize these disorders on the basis of different criteria—clinical syndromes, underlying renal pathology (including the percentage of glomeruli involved), molecular and cellular mechanisms underlying the injury,1 the localization of immune deposits,2 levels of serum complement,3 and specific antibodies involved.4 Moreover, although glomerular diseases may be named according to descriptive histologic findings, such as proliferative glomerulonephritis, these classic names do not distinguish between primary renal disease (i.e., disease limited to the kidneys) and glomerular involvement that is secondary to a multisystem disease.
To simplify matters, this topic classifies glomerular disease into two main groups, on the basis of the clinical presentation: nephritic syndrome (caused by glomerulonephritis) and nephrotic syndrome [see Table 1 and Figure 1]. Occasionally, a patient presents with features of both syndromes (nephritic-nephrotic syndrome). Two additional points should be noted. First, although patients typically present with nephritic or nephrotic syndrome, they may manifest an underlying systemic disease, such as systemic lupus erythematosus (SLE) or systemic vasculi-tis. Also, in early stages of glomerular diseases, patients may present with only hematuria or low-grade proteinuria before developing full-blown nephritic or nephrotic syndrome. Early recognition of the underlying disease process—with referral to a nephrologist—renal biopsy if indicated, and initiation of appropriate therapy are essential in preventing progressive renal injury. Second, patients may present with complications of glo-merular disease, such as hypertension, electrolyte and acid-base disturbances, and edema. Regardless of the clinical presentation, the physician should correlate the clinical syndrome with the underlying pathophysiologic events.
Epidemiology
Diseases of the glomerulus are the most common causes of end-stage renal disease worldwide. In the United States, the major glomerular diseases causing end-stage renal disease include diabetes mellitus (43%), hypertension (26%), and glomerulonephritis (9%). Worldwide, by far the leading cause of end-stage renal disease is glomerulonephritis, probably because of the prevalence of infectious diseases in developing countries. It is estimated that only 10% to 20% of patients with glomeru-lonephritis show clinical symptoms; thus, the prevalence of glomerular diseases may be underestimated.
Pathogenesis
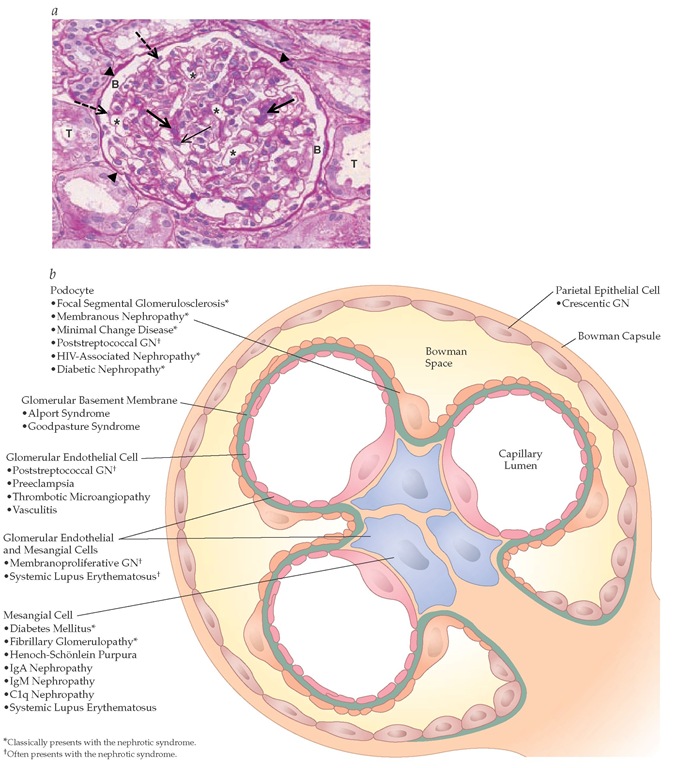
Each kidney contains approximately one million glomeruli. The glomerulus is a complex structure comprising a series of capillary loops that derive from the afferent arteriole and drain into the efferent arteriole. There are four glomerular cell types: mesangial cells, glomerular endothelial cells, podocytes (also called visceral epithelial cells), and parietal epithelial cells [see Figure 1a]. Each glomerular cell type serves distinct functions, and injury to each cell type results in a different renal histologic pattern and clinical picture [see Figure 1b].
The normal glomerular filtration rate (GFR) is about 80 to 120 ml/min. Approximately 30% of the plasma flow is filtered at the level of the glomerulus. The major barrier to filtration of plasma constituents is the glomerular capillary wall, which consists of glomerular endothelial cells, glomerular basement membrane (GBM), and podocytes.
Table 1 Clinical Presentation and Common Causes of Glomerular Disease
*Low complement.
 May present with the nephrotic syndrome.
May present with the nephrotic syndrome.
 May present with the nephritic syndrome.
May present with the nephritic syndrome.
ANCA—antineutrophil cytoplasmic antibody
GBM—glomerular basement membrane
GN—glomerulonephritis
The normal rate of urinary protein excretion is less than 150 mg/day, of which less than 20 mg is albumin. The normal filtration barrier limits passage of molecules by size and charge selectivity.6 The negative charge of the glomeru-lar endothelial cells, GBM, and podocytes limits the passage of anionic substances, such as albumin.7 Most glomerular diseases cause an alteration in both size and charge selectivity, which leads to proteinuria.
Glomerular diseases can be caused by a variety of mechanisms, including immune mediated, hemodynamic (e.g., hypertension, reduced renal mass), metabolic (e.g., diabetes, metabolic syndrome), and hereditary (e.g., defects of the GBM in Alport syndrome). The most common cause of glomerulonephritis is immune-mediated injury.8
Two mechanisms underlie antibody-mediated glomerular disease. One is the deposition of circulating antibodies directed against specific antigens in the glomerulus. The major example is Goodpasture syndrome, in which an antibody is directed against the noncollagenous portion of the a3 chain of type IV collagen in the GBM.9,10 Antibodies may be directed against an intrinsic glomerular antigen; for example, in idiopathic membranous nephropathy, the antibody is directed against an antigen on the podocyte.11 Alternatively, glomerular disease may result when antibody-antigen complexes circulating in the bloodstream are deposited in the subendothelial space or mesangium during the normal filtration process. Examples of such immune complex deposition include poststreptococcal glomerulone-phritis (PSGN) and membranoproliferative glomerulonephri-tis (MPGN) from hepatitis C.
Immune complexes generally activate either the alternative or the classical pathway of the complement cascade, thereby initiating injury.13 The site of the immune complex deposition determines which glomerular cell is injured and, therefore, which clinical manifestations develop.
In contrast to immune-complex mechanisms, certain glomer-ular diseases develop primarily from cell-mediated immunity.14 It is postulated that T cells sensitized to exogenous or endogenous antigens in the glomerulus recruit macrophages into the glomerulus, leading to a delayed-type hypersensitivity reaction. A classic example is minimal change disease, which has been postulated to occur when a T cell product injures podocytes and induces a permeability defect.
Diagnosis
Although renal biopsy remains the definitive diagnostic tool in glomerular disease, a thorough clinical evaluation is essential in determining the etiology and nature of these cases.
History
A carefully obtained history may help define a possible cause of glomerular disease. A family history of renal disease may suggest Alport syndrome, especially if affected individuals have hearing loss or another of the manifestations that are characteristic of this syndrome; there are also familial forms of IgA nephropathy, focal segmental glomerulosclerosis (FSGS), and hemolytic-uremic syndrome.
A number of glomerular diseases may be caused by drugs or toxins. For example, minimal change disease has been associated with the use of nonsteroidal anti-inflammatory drugs (NSAIDs) and interferon. Gold, penicillamine, NSAIDs, and mercury have been shown to cause membranous nephropathy. Thrombotic microangiopathy has been associated with the use of cy-closporine, tacrolimus, mitomycin C, and oral contraceptives.
Certain glomerular disorders are associated with malignancies. These include membranous nephropathy, which is associated with lung, breast, and gastrointestinal cancers; minimal change disease with Hodgkin disease; MPGN with non-Hodgkin lym-phoma; and amyloid with renal cell carcinoma.
Signs and symptoms
Patients with glomerular disease may be asymptomatic or may present with manifestations ranging from minimal findings to full-blown nephritic or nephrotic syndrome. Many patients have only mild to moderate proteinuria (150 mg to 3 g/24 hr) or microscopic hematuria (> two red blood cells [RBCs] per high-power field). Others present with painless gross hematuria. The classic example is hematuria immediately following an intercur-rent infection, which occurs in IgA nephropathy; onset of hema-turia 2 to 3 weeks after infection is typical of postinfectious glomerulonephritis. Patients with rapidly progressive glomeru-lonephritis (RPGN) present with rapid onset of renal failure over days or weeks with hematuria (from RBC casts) and proteinuria.
Patients with chronic glomerulonephritis generally have hypertension, renal insufficiency, and proteinuria. On renal ultra-sonography, the kidneys are somewhat smaller than normal and display increased echogenicity.
Renal biopsy
The ultimate diagnostic tool in many renal diseases is a renal biopsy, which is performed percutaneously with a spring-loaded biopsy needle, using local anesthesia, or surgically as an open biopsy with the patient under general anesthesia. Renal biopsies should be performed only after the patient has been evaluated by a nephrologist, but they also should be performed in a timely fashion, because early initiation of therapy is essential in preserving renal function and preventing further, potentially irreversible, injury to the kidney.
Although renal biopsy is generally a safe procedure, complications include hematoma, gross hematuria, arteriovenous fistula, and infection. In rare cases, renal biopsy leads to complications requiring surgical intervention.
Four situations mandate consideration of renal biopsy: (1) nephrotic syndrome (except in children, who may be assumed to have minimal change syndrome, and in diabetics, who are likely to have diabetic nephropathy); (2) renal disease in the setting of a systemic disorder (e.g., SLE, myeloma, amyloid, or vas-culitis); (3) acute renal failure in the setting of glomerular disease; and (4) renal abnormalities in renal transplant recipients (when there is a possibility of rejection or of recurrent or de novo glomerular disease). Some patients with nonnephrotic protein-uria, hematuria, and chronic renal failure may also benefit from a renal biopsy for diagnostic and prognostic purposes.
The renal biopsy establishes a diagnosis, determines whether disease is mediated by antibody or by complement, and assists in determining disease-specific therapy. The biopsy also provides important information on the degree of glomerular and interstitial fibrosis. The latter is particularly important as a prognostic index. The renal pathology report includes a description of the kidney by light microscopy, immunofluorescence, and electron microscopy.
Figure 1 (a) Light micrograph illustrating the different components of a normal glomerulus. Thick arrows illustrate mesangial cells; thin arrow illustrates glomerular endothelial cell; * illustrates capillary lumen; B illustrates Bowman space; dashed arrows illustrate podocytes; arrowheads illustrate Bowman capsule; and T illustrates tubule. (b) Schematic representation showing that injury to individual glomerular cell types or glomerular components causes specific forms of glomerular disease or glomerulonephritis.
Light Microscopy
Light microscopy describes glomerular cellularity—that is, whether the number of glomerular cells is normal or increased (hypercellularity). Often, light microscopy can distinguish which cell type (resident glomerular cells or infiltrating cells such as neutrophils) is increased; whether the GBMs are thickened and whether the capillary loops are patent, collapsed, or filled with material such as hyaline; and the presence or absence of glomerulosclerosis (scarring). Although the glomerulus is the primary site of injury in glomerular disease, the tubules and the interstitium must be carefully inspected because the degree of tubulointerstitial fibrosis is the best predictor of the prognosis in renal disease.
Light microscopy is also used to classify glomerular disease as focal or diffuse. If less than 50% of glomeruli are involved by the disease process, the disease is termed focal; if 50% or more glomeruli are involved, the disease is called diffuse. If a small portion (or segment) of an individual glomerulus is involved,the disease is described as segmental; if most of an individual glomerulus is involved, it is called global.
The presence of glomerular crescents can also be detected on light microscopy. Crescents are layers of cells (parietal epithelial cells, podocytes, lymphocytes, and macrophages) in the Bowman space, and their presence signifies severe disease.
Immunofluorescence
Immunofluorescent immunostaining determines the presence or absence of any underlying immune processes. Staining is directed against specific antibodies (e.g., IgG, IgA, and anti-GBM) and individual complement components (e.g., C3, C4, and C5b-9). The pattern of the immune components is also diagnostic. A granular pattern is typical of antigen-antibody complexes, such as in membranous nephropathy, whereas a linear pattern occurs in anti-GBM disease. The location of antibody or complement (e.g., in the mesangium in IgA nephropathy) also provides clues to the diagnosis. Immunostaining can determine the presence of matrix proteins (silver stain), amyloid fibrils (Congo red), and viral inclusions.
Electron Microscopy
Electron microscopy provides information about the presence and subcellular location of immune complexes (which are seen as electron-dense deposits), the degree of injury to glomerular cells, and the consistency of the basement membrane. Electron microscopy also detects fibrils and provides information on the ultrastructure of the kidney, such as podocyte effacement and flattening, which cannot be readily detected by light microscopy.