Historically, little attention has been devoted to studying female sexual function and dysfunction. A number of factors have contributed to this neglect. Gender bias remains strong.1 Cultural proscriptions, such as those regarding premarital intercourse, tend to be much more stringent for women. Women’s sexual pleasure is often valued less (exemplified in the common statement, "My husband’s needs come first") or not at all (demonstrated in its most extreme form by female circumcision, in which sensitive vulvar structures are surgically removed).2 Evaluation of sexual difficulties in women emphasized psychological origins, whereas biologic factors were relatively ignored.
Scientific investigation of female sexuality has been hampered by numerous methodological challenges. Arriving at a precise definition of female sexual dysfunction, for example, has proved difficult because sexual difficulties reported by women are not discrete, tending to occur together. Specific diagnostic criteria, such as duration of symptoms and degree of distress, continue to be debated. Sexual response is more difficult to evaluate objectively in women than in men, and until recently, few reliable animal models existed.
For all of these reasons, funding to support research on female sexuality has been suboptimal. Nevertheless, knowledge about female sexuality has expanded rapidly during the past decade. Although further study is needed to throroughly address remaining questions and controversies, there are now sufficient data to guide rational evaluation of women who present with sexual concerns.
As in men, sexual problems in women impact quality of life, general functioning, and adaptation to illness. Levels of distress associated with sexual dysfunction range from mild unhappi-ness, frustration, or a sense of sexual inadequacy to a more pervasive loss of self-esteem that can have profound effects on intimate relationships, as well as functioning in other social and occupational realms.3 Sexual complaints can be important clues to the presence of an underlying illness, such as depression. Sexual side effects of medications are common and may result in lack of compliance if they are not adequately addressed. For these reasons, it is imperative for clinicians to become adept at addressing patients’ sexual concerns. However, many practitioners lack formal training in this area and thus are unsure of what questions to ask and what to do when a sexual problem is identified. This topic provides a practical approach to the evaluation and management of sexual dysfunction in women.
Epidemiology
In the Pfizer Global Study of Sexual Attitudes and Behaviors, a survey of over 27,500 persons in 29 countries, 63% of women (and 83% of men) 40 to 80 years of age described sex as extremely, very, or moderately important.4 It is reasonable to assume that younger men and women find sex to be equally if not more important.
Large population-based studies in several countries have examined the prevalence of sexual difficulties in women and men.
In all of the studies, a higher percentage of female respondents than male respondents reported experiencing sexual difficulties: 43% versus 31% in the United States,5 54% versus 35% in the United Kingdom,6 30% versus 27% in Sweden,7and 71% versus 46% in Australia.8 The wide variation in these numbers may reflect differences in the specific questions that were asked, the time intervals that were studied, the demographics of the study populations, and whether or not unpartnered persons were included. The numbers may be inflated in that not all persons who report having sexual difficulties consider themselves to have a sexual dysfunction: only one third to one half of women who report having decreased sexual desire or response believe they have a problem or feel distress for which they would like help.9 However, even when personal distress and the desire for intervention are factored in, the prevalence of sexual problems in women remains high (10% to 35% of women in these studies).
Some, but not all, studies of sexual function report an association between older age and lower desire; however, when the relationship between low interest and distress over sexual difficulties is taken into account, the age factor largely disappears.9 Although menopause can be associated with decreased lubrication, vaginal dryness, and discomfort during sexual penetration, pain during sex seems to be significantly more common in younger women than in older ones.5 In a large random telephone survey done in Australia, which included 8,282 women and 8,510 men 16 to 59 years of age, the most common problem cited by both women and men was lack of sexual interest.8 Women and men felt equally anxious about their ability to perform. Women were more likely than men to report being unable to achieve orgasm, not finding sex pleasurable, experiencing physical pain during intercourse, and worrying during sex that their body looked unattractive. Few studies have examined the effect of factors such as ethnicity and sexual orientation on rates of sexual satisfaction and sexual difficulties in women.
Female Sexual Function
Female sexual function is far more than a simple biologic response to a stimulus. When a woman experiences sexual difficulty, there is almost never just one cause; a multifactorial etiology is the rule. Similarly, there is usually no quick fix. This may help explain why medications such as sildenafil and other phos-phodiesterase (PDE5) inhibitors, which rapidly restore sexual function in many impotent men, are not particularly useful in most women.10,11 To fully understand the nuances in each case, it is crucial to identify and address all of the factors that contribute to the patient’s lack of satisfaction with her sex life. These factors can be readily enumerated if the clinician examines each case in two ways: first, from the perspective of a biopsychosocial model of sexual function and, second, by examining which functional domains (i.e., desire, arousal, orgasm, satisfaction, absence of pain) are affected.
Biopsychosocial model of female sexual function
Normal female sexual function involves successful integration of key factors in psychological, interpersonal, and biologic realms [see Figure 1]. For example, women raised in societies in which discussion of sex is taboo are often ignorant about genital anatomy, sexual needs, and techniques. Young women whose families have strong expectations regarding sexual orientation and virginity may feel anxiety, confusion, or shame if their evolving sense of self turns out to be discordant with the values of their families. Finally, gender roles shape power dynamics in relationships and likely affect the degree to which different societies tolerate sexual coercion and violence. A pattern of sexual inhibition and avoidance often follows sexual trauma; disempowered women typically find it difficult to communicate sexual needs to their partner or to advocate for safer sex.
Figure 1 The biopsychosocial model posits that overlapping physiologic, psychosocial, and relational factors influence sexuality.
In addition to culture and contextual background, studies show considerable variation with respect to how women rate the importance of sex, the specific sexual practices they choose to participate in, the frequency of sexual activity they feel is optimal, and the intensity and duration of stimulation they need to achieve arousal and orgasm.12 What would be a satisfying sex life for one woman might seem woefully inadequate to another. Clearly, the only person who can decide she has a sexual problem is the patient herself. Diagnosis of a sexual dysfunction should never be made unless a problem is persistent and causes distress and unless the patient articulates a desire for further evaluation.13
Models of human sexual response
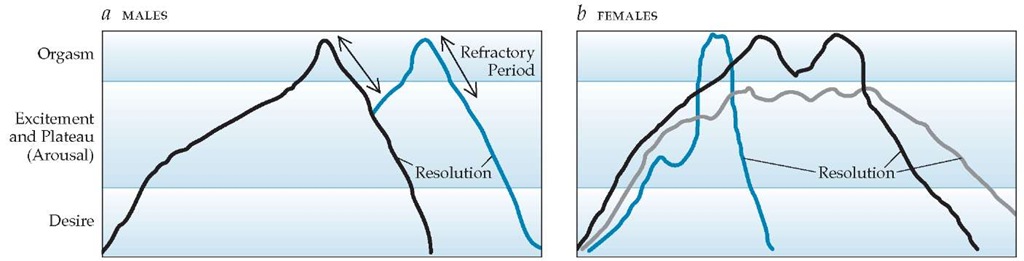
Traditional models of male and female sexual response were initially described by Masters and Johnson in the 1960s and embellished later by Helen Kaplan [see Figure 2].14,15 In these linear models, events proceed in an orderly, stepwise manner; desire is a necessary first step; and orgasm is the explicit goal, although it may not always be attained. An important difference between the sexes is that orgasm in the male is followed by a longer refractory period; multiple sequential orgasms are more readily achieved in women.
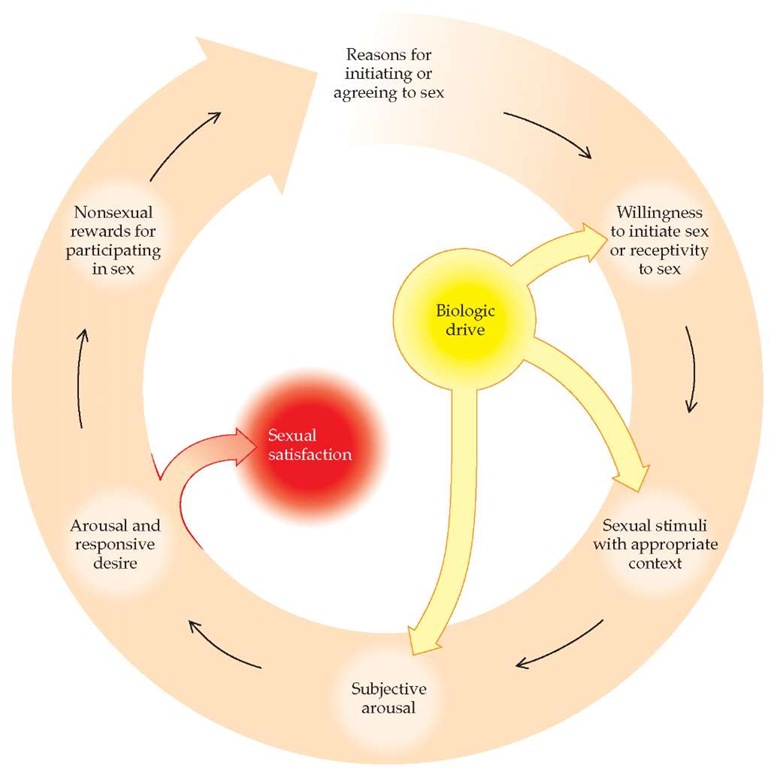
Basson and colleagues developed a new model of sexual response in an attempt to convey more aptly the complexity of female sexual function.16 This model is circular and contains multiple feedback loops through which sexual desire and arousal may be intensified or inhibited [see Figure 3]. Spontaneous desire is not a necessary factor to enter the cycle; simply feeling emotionally close and engaging in intimate touching can lead to arousal. This more realistic model acknowledges the fact that subjective response (emotional closeness to one’s partner during sexual activity) can be as important for some women as physical response (attainment of orgasm).
Figure 2 Traditional models of human sexual response are linear.14,15 In males (a), orgasm is followed by a short refractory period, after which further stimulation (blue line) can again lead to arousal and a second orgasm. Females (b) may experience a single-orgasm event (blue line), multiple orgasms (black line), or a plateau without orgasm (gray line).
Figure 3 Female sexual response is most accurately described by a circular model containing multiple feedback loops.139 At several points in the cycle, sexual response can be either inhibited or stimulated by cognitive influences (e.g., negative thoughts can interrupt the response; erotica or fantasy can enhance the response). Sexual satisfaction can occur with or without orgasm. Nonsexual rewards may include emotional intimacy, feelings of well-being, or avoiding negative consequences for not participating in sex.
Structure and erogenicity of the female genitalia
Erogenous external genital structures in women include the clitoris, labia majora and minora, vaginal introitus, and anus. Of these, the densely innervated and highly sensitive clitoris is exceedingly important: arousal proceeds most readily and orgasms are strongest when the clitoris is stimulated.17 Indeed, an excessive focus on penile-vaginal intercourse without clitoral stimulation is a chief cause of anorgasmia in women.
Internal genital structures involved in female sexual response include the vagina, cervix, and uterus. Of these, stimulation of the anterior vagina seems to be most pleasurable. Popular literature describes a so-called G-spot, or Grafenberg spot, an allegedly highly erogenous area located along the lower third of the anterior vaginal wall.18 Although few studies provide objective evidence supporting the existence of the G-spot, stimulation of this area is purported to result in a kidney bean-size area of swelling and to be associated with high levels of arousal and powerful orgasms. Similarly, the existence of female ejaculation has been postulated because of the observation that fluid is released during orgasm in some women. Whether this fluid is simply urine or might represent a true ejaculate, such as emission of secretions from female paraurethral glands (analogous to the male prostate), is not clear from available data.18
Despite its proximity to the major pelvic or paracervical ganglion, the cervix itself is a relatively insensitive structure.19 There are numerous theoretical reasons why removal of the uterus might be expected to interfere with sexual response, including anatomic changes (shortening of the vaginal vault and scar formation in the vaginal cuff), surgical damage to pelvic nerves and blood vessels, secondary hormonal changes,20 and the possibility that uterine contraction itself contributes substantively to orgasmic pleasure. Although studies of sexual function after hysterectomy are fraught with methodological problems, the preponderance of evidence suggests that detrimental effects of either total or supracervical hysterectomy are rare and that preoperative sexual function is the most important predictor of postoperative sexual satisfaction.21
Biochemistry and metabolism of sex steroids
Sex steroids and their receptors play a key role in the maturation, maintenance, and function of the tissues that are involved in female sexual response. The modulating effects of estrogen and androgen help explain the changes in sexual function that are associated with changes in the levels of these hormones, whether the hormones are endogenous (as with changes that occur during puberty, within each menstrual cycle, during pregnancy and post partum, during lactation and after weaning, and with surgical or natural menopause) or exogenous (i.e., given as contraceptives or fertility drugs or for hormone replacement). Other hormones, including progesterone, oxytocin, and pro-lactin, probably also have important effects.
Synthesis of both estrogen and androgen takes place in the ovaries, adrenal glands, and peripheral tissues. Estradiol predominates before menopause. After menopause, estrogen is made extragonadally via aromatization of ovarian and adrenal androgens, and estrone predominates. The ovaries produce an-drostenedione, testosterone, and a small amount of dehy-droepiandrosterone (DHEA). The adrenals produce androstene-dione and DHEA sulfate (DHEAS). Both DHEA and DHEAS are converted in brain, bone, and adipose tissue to androstene-dione or testosterone, which can then be either converted by 5-reductase to dihydrotestosterone (DHT) or aromatized to es-trone or estradiol. Only free testosterone and DHT can bind to receptors and are therefore biologically active.
Before menopause, 25% of a woman’s testosterone is produced in the ovaries, 25% in the adrenals, and the remaining 50% in peripheral tissues. Circulating androgen levels peak during the third decade of life, then decrease slowly with age because of reduced adrenal synthesis.22 Diurnal and menstrual cycle-linked changes in testosterone and androstenedione also occur, with levels highest in the morning before 10 A.M. and during the middle third of the menstrual cycle.22 Stromal cells of the ovary continue to make androgen precursors and testosterone after natural menopause; the amount of bioavailable testosterone may actually rise slightly during the first few years of the menopausal transition because of lower serum estrogen and, therefore, lower sex hormone-binding globulin (SHBG) levels.23 Surgical menopause presents a dramatically different situation: bilateral salpingo-oophorectomy can lead to a sudden 50% decline in circulating levels of androstenedione and testosterone, with the effect being most pronounced in younger women.24 Compromised ovarian function from premature ovarian failure, chemotherapy, pelvic radiotherapy, or administration of gonadotropin-releasing hormone agonists is also associated with low circulating androgen levels, as are adrenal insufficiency or adrenalectomy, glucocorti-coid treatment (which suppresses pituitary adrenocorticotropic hormone secretion), and administration of exogenous estrogen (which increases SHBG levels and suppresses pituitary luteiniz-ing hormone secretion).25
Neurobiology of female sexual response
Studies of the anatomy, physiology, and pathophysiology of female sexual function and dysfunction are limited. Much of what is known, or inferred, is extrapolated from animal studies, primarily in rodents, and drawn by analogy from studies in men.19
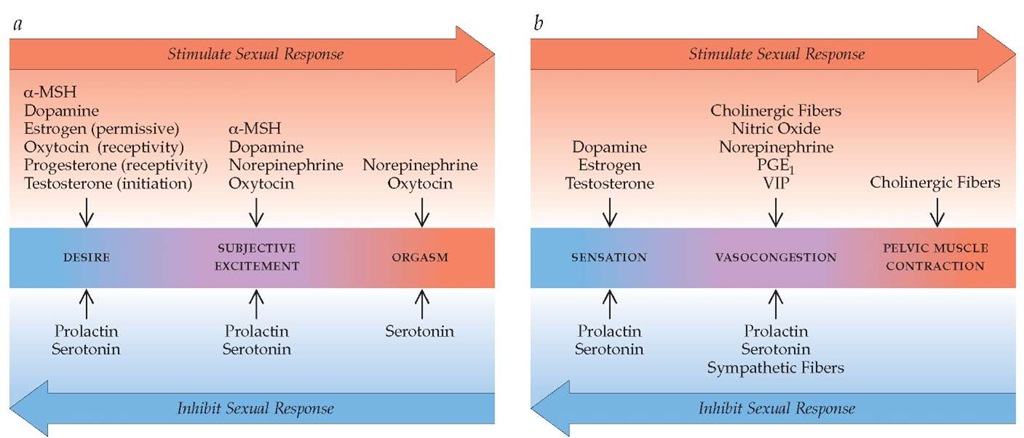
Normal female sexual function involves intact neural, vascular, and muscular circuitry; complex interactions between multiple neurotransmitter systems; and critical modulating influences from the endocrine system. Numerous neurotransmitters, bioac-tive substances, and sex steroids appear to be involved. These include, but are probably not limited to, dopamine, norepineph-rine, serotonin, acetylcholine, nitric oxide, vasoactive intestinal peptide (VIP), prostaglandin E1 (PGE1), estrogen, testosterone, progesterone, oxytocin, and prolactin. Emerging evidence suggests that a-melanocortin-stimulating hormone (a-MSH) may also play an important role.26 Some of these substances act centrally, in the brain and spinal cord, whereas others have peripheral sites of action (e.g., arteries, peripheral nerves, and the pubococcygeal muscles) [see Figure 4]. Some neurotransmitters have effects at both central and peripheral locations, but the nature of these effects (i.e., excitatory versus inhibitory) is not always the same at these two locations. For example, central norepinephrine activity appears to have stimulatory effects on female sexual response, whereas peripheral effects tend to be largely inhibitory. This has important implications for the design of pharmacologic therapies for female sexual problems. Because peripheral responses (e.g., vasocongestion) and observable behaviors (e.g., lordosis as a measure of proceptivity and receptivity in female rats) are easier to measure than central events, research to date has concentrated more on peripheral mechanisms than on central ones. The neuro-physiology of each phase of the female sexual-response cycle— desire, arousal, and orgasm—can be considered separately.
Neurobiology of Desire (Libido)
Desire can be defined as a mental state created by external and internal stimuli that induce an urge to participate in sexual activity.19 Manifestations of increased desire include sexual thoughts or fantasies and the motivation to initiate, or the willingness to be receptive to, sexual activity. It is useful to consider three non-mutually exclusive aspects of sexual desire: drive, motivation, and beliefs/values.
Sex drive has biologic roots and appears to be mediated by the excitatory action of dopamine and modulating effects of sex steroids in the mesolimbic system of the brain.27 Higher-order cortical processes likely provide excitatory and inhibitory influences on lower cortical centers. Dopamine enhances sex drive and the wish to continue sexual activity once sexual stimulation has been initiated; these effects are inhibited by serotonin. Estrogen seems to have a small permissive effect, whereas testosterone and progesterone appear to influence initiation of sexual activity and receptivity to partner approach, respectively.27 Serum prolactin concentrations increase after orgasm in men and women, and chronic hyperprolactinemia is associated with reduced libido in both sexes.28 These observations suggest that prolactin may be a regulatory factor that signals central nervous system centers involved in the initiation or control of sexual behavior. Although sexual pathways in female humans need to be further elucidated, a-MSH agonists have been found to selectively stimulate so-licitational behaviors in female rats and enhance desire and stimulate erection in men with psychogenic erectile dysfunction.26
Sexual motivation is fueled by anticipation of a risk or reward associated with initiation of or participation in sexual activity. Incentives are diverse and may include the following: feeling close to one’s partner, giving or experiencing sexual pleasure, relieving tension, becoming pregnant, or exchanging sexual favors for tangible and intangible gifts. Conversely, the expectation, based on negative past experiences, of pain or injury resulting from sexual activity serves as a major disincentive.
Beliefs/values include unique personality and cultural factors that exert either excitatory or inhibitory influences on the wish to engage in sexual activity. Motivation and beliefs/values probably affect libido via excitatory and inhibitory cortical influences on lower cortical structures; further study is needed to elucidate specific pathways.
Knowledge of the basic neurophysiology of libido helps predict the effects of various exogenous substances. As expected, libido seems to be increased by dopamine enhancers, such as amphetamines and norepinephrine-dopamine reuptake inhibitors (NDRIs) (e.g., bupropion). Conversely, substances that reduce dopamine activity diminish libido: examples include D2 dopamine receptor blockers and selective serotonin reuptake inhibitors (SSRIs). Hyperprolactinemia (caused by pituitary macro-adenomas, lactation, or use of so-called typical antipsychotic drugs), intoxication with CNS depressants such as alcohol, and use of antiandrogens also reduce libido.
Figure 4 The neurobiology of female sexual response includes both central and peripheral events.27 Although these may be visualized as occurring sequentially, in reality, the physiologic and biochemical components of arousal may occur simultaneously and to some extent independently. Whether sexual desire, arousal, and orgasm are enhanced or inhibited depends on the coordinated activity of numerous bioactive substances, as well as the presence or absence of key environmental influences.
(a) Central events are critical in the emergence of sexual desire and subjective excitement (arousal) and play an important role in orgasm. Increased levels of serotonin can result in inhibition of this response through reduction of dopamine and norepinephrine levels in the brain.
(b) Peripheral events include genital sensation, vasocongestion, and muscle contraction. The baseline state of the genital vasculature is tonic vasoconstriction, which is maintained by outflow from sympathetic fibers. In response to stimulation, release of vascular mediators (e.g., nitric oxide, prostaglandin E1 [PGE1], and vasoactive intestinal peptide [VIP]) promotes dilation of vessels and tissue vasocongestion. Both vasoconges-tion and pelvic muscle contractions are mediated by the release of acetylcholine from cholinergic fibers. Increasing feedback along afferent pathways to the spinal cord culminates in a spinal reflex arc that produces pelvic muscle contractions; transmission of these impulses cephalad to pleasure centers in the brain produces the sensation of orgasm.
Fortunately, decreased libido does not have to relegate a woman to an unsatisfactory sex life. Many women whose spontaneous level of desire is reduced or absent retain the capacity for arousal and even orgasm by strong cognitive motivation to engage in sexual activity and skillful sexual-stimulation technique.
Neurobiology of Arousal
Sexual arousal includes both subjective excitement (i.e., awareness of, comfort with, and appreciation of erogenous stimulation) and objectively measurable signs (both nongenital and genital) of physiologic arousal.27 It is important to distinguish between subjective and objective arousal, because studies show that women often experience measurable physiologic arousal in the absence of a subjective sense of excitement or pleasure.13 Indeed, the feeling of sexual arousal in women seems to be heavily dependent on cognitive processing of stimulus meaning and content,29 as opposed to being related purely to peripheral vaso-congestive and neuromuscular events.
Subjective arousal is a central event that appears to be mediated by excitatory effects of both dopamine and norepineph-rine; oxytocin, which is elevated throughout the follicular phase of the menstrual cycle, may also play a stimulatory role.30,31 The effects of dopamine and norepinephrine are inhibited by serotonin; prolactin also has a negative effect on subjective excitement.
Genital sexual arousal appears to be mediated by spinal reflexes.31 One such spinal reflex is the bulbocavernosus reflex, in which stimulation of light-touch pudendal sensory fibers activates pudendal motor neurons, resulting in contraction of striated perineal muscles. Activation of this reflex contributes to development of the orgasmic platform and strengthens urinary continence during arousal. The bulbocavernosus reflex can be evaluated during physical examination to check both sensory and motor function of the pudendal nerve. Another spinal reflex involves vaginal and clitoral cavernosal autonomic nerve stimulation, which results in clitoral, labial, and vaginal engorge-ment.19 The efferent and afferent arms of these spinal reflexes have been relatively well defined in men; pathways in women are probably similar. Efferent facilitatory parasympathetic output arises in the sacral parasympathetic nucleus and is conveyed to the vagina and clitoris by the pelvic nerve. Efferent inhibitory sympathetic output arises in the dorsal gray commissure and the intermediolateral cell column at the thoracolumbar level and travels to the genitalia via the hypogastric nerve and the para-vertebral sympathetic chain. The activity of the spinal nuclei is controlled both by descending projections from multiple supra-spinal sites and by sensory afferents from the genitalia that are conveyed by the pudendal, hypogastric, pelvic, and vagus nerves. Serotoninergic projections from the brain to the spinal cord appear to inhibit the induction of genital arousal by afferent peripheral stimulation; noradrenergic projections also exist, but their functional significance is not fully understood.31
In the basal state, clitoral corporal and vaginal smooth muscles maintain contractile tone under noradrenergic mediation.19 During sexual stimulation, neurogenic and endothelial release of nitric oxide, produced by the action of nitric oxide synthase on L-arginine, triggers a rise in cyclic guanosine monophosphate (cGMP) in postjunctional cells, causing calcium influx to vascular smooth muscle in the clitoris and vagina, vasodilatation, and tissue engorgement.19 The result is extrusion of the glans and enhanced sensitivity. The vaginal epithelium continuously reab-sorbs sodium from submucosal capillary plasma transudate. During sexual stimulation, release of nitric oxide and VIP induce increased capillary inflow; sodium reabsorption becomes overwhelmed; and 3 to 5 ml of vaginal transudate is produced, enhancing lubrication during sexual activity.19 In addition to nitric oxide and VIP, a number of other vasoactive substances, including PGE1, have been found in the clitoris and vagina; all deserve further study.32 Cholinergic fibers innervate vascular smooth muscle in the vagina and may help mediate vaginal engorgement during arousal; however, the chief role of acetylcholine in the periphery is to increase pelvic striated muscle contractility.33 Estrogen plays a critical role in maintaining vaginal tissue integrity and has local regulatory effects on nerve transmission (thereby increasing genital sensation) and mediators of vasocon-gestion (thereby enhancing genital blood flow and lubrication).34 Preliminary evidence suggests that androgens may also affect genital sensation, genital hemodynamics, and mucin production (a minor component of sexual lubrication).34 Peripherally, serotonin acts via 5-hydroxytryptamine-2A (5-HT2A) receptors to reduce genital sensation and to inhibit nitric oxide synthase.35 Exogenous substances that increase genital arousal include estrogens; pPDE5 inhibitors; PGE1 agonists; and alpha blockers. PDE5 inhibitors potentiate the effects of the nitric oxide pathway by blocking the degradation of cGMP.36 Substances that reduce genital arousal include antiestrogens, SSRIs, and anticholinergic medications.