Hilar and Mediastinal Disorders
Normal anatomy of the hila and mediastinum
The mediastinum is the intrathoracic compartment situated between the two lungs. It is bordered anteriorly by the sternum, posteriorly by the vertebral column, and laterally by parietal pleura. It extends from the thoracic inlet to the superior surface of the diaphragm. Several important structures are contained within or pass through the mediastinum, including the heart and great vessels, the esophagus, the trachea and mainstem bronchi, lymphatic vessels and nodes, and nerves.
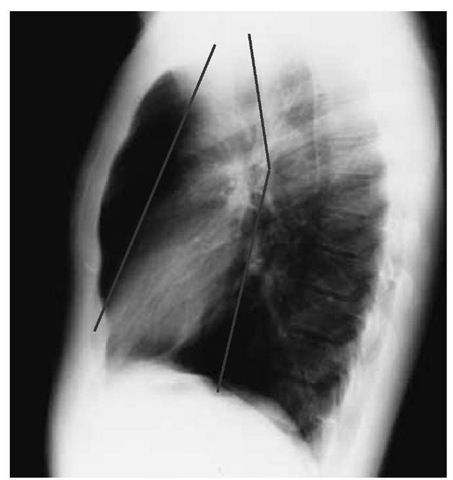
It is convenient to divide the mediastinum into three parts on the basis of imaginary coronal sections [see Figure 10]. The anteri- or mediastinum (i.e., the portion anterior and superior to the anterior surface of the pericardium) contains the thymus, lymph nodes, and mesenchymal tissue. The middle mediastinum consists of the heart and pericardium, the major vessels as they enter and leave the heart, the trachea and main bronchi, lymph nodes, and portions of the phrenic and vagus nerves. Between the posterior aspect of the pericardium and the vertebral column lies the posterior mediastinum, which contains the descending aorta, the esophagus, the thoracic duct, lymph nodes, and a portion of the vagus nerve. The sympathetic nerve chain, which runs in the costovertebral gutter, is conventionally included in the posterior mediastinum.
Figure 10 Lines drawn on this normal lateral chest x-ray mark the theoretical division of the mediastinum into anterior, middle, and posterior compartments. Focal abnormalities of the mediastinum can be identified as occurring predominantly in one compartment or another, facilitating differential diagnosis.
As blood vessels, airways, and nerves leave the mediastinum and pass into the lungs, they form the pulmonary hila. On chest radiographs, the hilar shadows are composed primarily of branches of the pulmonary arteries. The right pulmonary artery bifurcates just before entering the hilum as the truncus anterior artery (superior branch) and the right interlobar artery (inferior branch). The left pulmonary artery divides within the hilum into superior branches and the larger left interlobar artery. In 97% of normal persons, the left hilum is positioned superior and posterior to the right hilum; displacement from this normal position may be caused by mass lesions or lobar atelectasis. Central bron-chopulmonary lymph nodes that are important for drainage of the lung parenchyma are situated within the pulmonary hila along the bronchial tree, especially within bifurcations.
Hilar enlargement
The most common causes of enlargement of the hilar shadows on chest radiographs are vascular engorgement and adenopathy.
Enlargement Caused by Vascular Engorgement
Vascular engorgement may result from increased blood flow in the pulmonary circulation, as in atrial septal defect with left-to-right intracardiac shunt, or from pulmonary arterial hypertension of any cause. The caliber of the pulmonary arteries can be assessed by measurement of the transverse diameter of the right interlobar artery on a posteroanterior radiograph; in normal persons, this value is usually less than 16 mm. In rare cases, localized aneurysmal dilatation of the pulmonary arteries occurs; this condition is referred to as the Hughes-Stovin syndrome.
On plain chest radiographs, it is sometimes difficult to distinguish vascular from nodal enlargement of the hila. Pulmonary angiography was formerly needed to make this distinction. Currently, however, contrast-enhanced CT scanning of the chest can effectively identify vascular structures in the hila without the need for invasive procedures. Occasionally, there is a strong contraindication to the use of intravenous contrast agents in some patients; in such cases, magnetic resonance imaging, which does not require the use of contrast agents, can be used.
Enlargement Caused by Adenopathy
Hilar lymph node enlargement may be unilateral or bilateral. Unilateral lymphadenopathy may accompany virtually any pneumonia, although it is most characteristic of granulomatous infections (e.g., tuberculosis and atypical mycobacteriosis, histo-plasmosis, and coccidioidomycosis) and certain atypical pneumonias (e.g., Mycoplasma infections, tularemia, pertussis, and psittacosis). Neoplastic enlargement of hilar lymph nodes usually results from spread of bronchogenic carcinoma; extrathoracic cancers that metastasize to the hilar and mediastinal lymph nodes include cancers of renal cell origin, which are implicated especially often, and cancers of the breast and GI tract. Hodgkin disease and other lymphomas may also cause unilateral or asymmetrical hilar adenopathy. Finally, about 1% to 3% of patients with sarcoidosis have unilateral hilar adenopathy.
A common diagnostic challenge is the evaluation of a patient with bilateral hilar adenopathy. This condition is often found in association with mediastinal adenopathy and sometimes in association with parenchymal infiltrates. Nonspecific symptoms of cough, chest pain, dyspnea, or malaise may have prompted the chest radiograph, or bilateral hilar adenopathy may have been detected incidentally on a chest radiograph obtained for unrelated reasons. The most common etiology, especially in patients between 20 and 40 years of age, is sarcoidosis. The differential diagnosis of bilateral hilar adenopathy includes the following: (1) lymphoma, which is usually accompanied by extrathoracic manifestations, such as systemic symptoms, peripheral adenop-athy, and anemia; (2) metastatic cancer, in which the primary malignant disease is most often known; (3) chronic granuloma-tous infections, such as tuberculosis or histoplasmosis, in which the adenopathy is more commonly unilateral; and (4) berylliosis, which can precisely mimic sarcoidosis but which can be readily diagnosed with a careful occupational history.
In a retrospective analysis of 100 cases of bilateral hilar adenopathy not caused by infection, it was found that patients who were asymptomatic and had a normal physical examination or those who had erythema nodosum or uveitis as the only manifestation of their disease invariably had sarcoidosis. On the other hand, it was found that patients who were symptomatic or patients with other abnormal physical findings (e.g., peripheral adenopathy, hepatomegaly, or splenomegaly) in some cases had sarcoidosis and in other cases had neoplastic node involvement.
The approach to diagnosis in patients with bilateral hilar adenopathy depends on the relative likelihood of the various diagnostic probabilities, as determined by clinical assessment. Patients strongly suspected of having sarcoidosis may be observed with serial chest radiographs or may undergo transbronchial lung biopsy via the fiberoptic bronchoscope for tissue confirmation (i.e., identification of noncaseating granulomas). In patients with granulomatous infection, CT scanning can indicate the presence of active infection when areas of low attenuation (necrosis), peripheral rim enhancement, and larger nodes are seen, whereas inactive infection is suggested by smaller nodes, homogeneous density, and calcification.81 When lymphoma is suspected, lymph node biopsy by mediastinoscopy should provide diagnostic material. In patients suspected of having carcinoma of the lung, an increase in the uptake of fluorodeoxyglucose by the thoracic lymph nodes, as shown through use of positron emission tomography, indicates high risk of malignancy.82 The definitive diagnosis of cancerous lymph nodes can be determined with a high yield by needle aspiration of the hilar mass under fluoroscopic, CT, or transesophageal endosonographic guidance.83 It should be noted, however, that direct histologic analysis of hilar lymph nodes, which might be necessary in cases of lymphoma in which there is no mediastinal involvement, requires at least a limited anterior thoracotomy to obtain a tissue sample of adequate size.
Enlargement Caused by Calcified Lymph Nodes
Calcified hilar lymph nodes most often indicate previous granulomatous infection, especially tuberculosis or histoplasmo-sis. Hilar and mediastinal lymph node calcification in patients with silicosis may appear in a highly characteristic pattern referred to as eggshell calcification, in which calcium outlines only the perimeter of the lymph nodes. Sarcoidal lymph nodes rarely become calcified.
Acute mediastinitis
Acute bacterial mediastinitis usually occurs as the consequence of esophageal perforation and the subsequent release of acidic gastric juices, often along with anaerobic bacteria, into the mediastinum. Rupture of the lower esophagus from violent vomiting is referred to as Boerhaave syndrome; free gas in the mediastinum (i.e., pneumomediastinum) and pleural effusion or pneumothorax (which is usually left sided) are common manifestations of this syndrome. Esophageal rupture may also occur as a complication of foreign-body ingestion, esophageal carcinoma, penetrating or blunt chest trauma, and medical procedures that dilate the esophagus. Occasionally, infection spreads to the mediastinum from adjacent sites; empyema, lung abscess, pericarditis, and retropharyngeal abscess84 may all be complicated by acute mediastinitis. Mediastinitis can also be a complication of sternotomy for cardiac or thoracic surgery.85 Finally, a mediastinal bronchogenic cyst may become infected and discharge its contents into the mediastinum.
Patients who have acute mediastinitis are usually acutely and severely ill. Symptoms of the disorder include fever, dysphagia, and a lancinating chest pain. The chest radiograph may reveal, in addition to mediastinal widening, such abnormalities as the presence of pneumomediastinum and pleural effusions.
Treatment consists of the administration of broad-spectrum antibiotics, including antibiotics that are effective against anaerobic bacteria, because the infections are usually polymicrobial.86 In addition, open surgical drainage is required in most cases.87 Per- cutaneous catheter drainage may be successful in some cases of limited esophageal leakage.
|
Table 3 Causes of Mediastinal Widening88 |
|
Diffuse Mediastinal Widening |
|
Acute mediastinitis |
|
Hemorrhage |
|
Lipomatosis |
|
Fibrosing mediastinitis |
|
Anterior Mediastinal Masses |
|
Thymus disorders |
|
Thymoma |
|
Thymic cyst |
|
Thymolipoma |
|
Thymic hyperplasia |
|
Thymic carcinoma |
|
Thymic carcinoid |
|
Teratoma and dermoid cyst |
|
Intrathoracic goiter or thyroid carcinoma |
|
Lymphoma |
|
Parathyroid masses |
|
Malignant germ cell neoplasms |
|
Endodermal sinus tumor |
|
Seminoma |
|
Primary choriocarcinoma |
|
Mixed germ cell tumor |
|
Mesenchymal neoplasms |
|
Lipoma |
|
Fibroma |
|
Hemangioma |
|
Lymphangioma (cystic hygroma) |
|
Middle Mediastinal Masses |
|
Lymphoma |
|
Carcinoma of the trachea |
|
Carcinoma metastases to the lymph nodes |
|
Granulomatous mediastinitis |
|
Bronchogenic cyst |
|
Pleuropericardial cyst |
|
Diaphragmatic hernia (through foramen of Morgagni) |
|
r o ^ o o o ‘ Benign lymph node hyperplasia (Castleman disease) |
|
Vascular dilatation |
|
Posterior Mediastinal Masses |
|
Neurogenic tumors |
|
Cysts |
|
Neurenteric |
|
Gastroenteric |
|
Thoracic duct |
|
Esophageal neoplasms and diverticula |
|
Diaphragmatic hernia (through foramen of Bochdalek) |
|
Diseases of the thoracic spine |
|
Extramedullary hematopoiesis |
Mediastinal widening and mass lesions
A great diversity of benign and malignant lesions may cause the mediastinum to have an abnormal appearance on the chest radiograph. Occasionally, a specific set of findings may prompt a search for a mediastinal lesion. For example, the muscle weakness of myasthenia gravis may prompt a search for associated thymomas. Alternatively, the constellation of abnormalities suggesting the superior vena cava syndrome (e.g., headaches, jugular venous distention, engorgement of the head and neck, and a prominent collateral venous pattern across the upper thorax) may prompt a search for an obstructing lesion [see 12:VIII Lung Cancer and 12:XII Oncologic Emergencies]. In some patients, the mediastinal disorder induces nonspecific symptoms referable to the thorax, such as cough, dyspnea, chest pain or pressure, hemoptysis, dysphagia, hoarseness, or wheezing. Most often, however, diagnostic evaluation is prompted by an asymptomatic ra-diographic finding.
The differential diagnosis of abnormal radiographic opacities within the mediastinum includes benign and malignant neoplasms, cysts, vascular abnormalities, ectopic thyroid tissue, granulomatous diseases, and several other disorders.88 It is useful to distinguish abnormalities that cause diffuse widening of the mediastinum from those in which the abnormality is confined primarily to the anterior, middle, or posterior mediastinum [see Table 3]. Selected examples of each category will be presented.
With the use of intravenous contrast agents, CT scanning can distinguish vascular structures, fat density, cysts, and calcifications from soft tissue density. Often, benign mediastinal lesions can be identified with reasonable certainty by the CT image alone; nonneoplastic lesions include those associated with mediastinal lipomatosis, congenital cysts, vascular aneurysms, diaphragmatic hernias, and intrathoracic goiters. In other cases, CT scanning can be used with a high degree of sensitivity and specificity to guide the biopsy of suspected malignant lesions.89 MRI may provide advantages over CT in cases suspected to be vascular.90
Diffuse Mediastinal Widening
Diffuse mediastinal widening may occur acutely or chronically. Acute widening of the mediastinal shadows may result from an acute mediastinitis or from mediastinal hemorrhage. Important causes of chronic mediastinal widening include mediastinal lipomatosis and mediastinal granuloma and fibrosis.
Mediastinal Lipomatosis
Mediastinal lipomatosis is a consistently asymptomatic condition in which excess fat tissue is deposited between and around mediastinal structures; it is found in some patients with Cushing disease, iatrogenic Cushing syndrome, or obesity. The only significance of mediastinal lipomatosis is that it must be distinguished from other, more serious causes of mediastinal widening. CT scanning of the thorax readily establishes the diagnosis by identifying material of fat density deposited diffusely through the mediastinum.
Mediastinal Granuloma and Fibrosis
Mediastinal lymph node involvement is common in patients with chronic granulomatous infections, such as histoplasmosis and tuberculosis. Infrequently, an excessive fibrotic response develops around a caseous focus within lymph nodes. In some cases, fibrosis causes a capsule 2 cm or greater in thickness to form around a caseous focus, giving rise to a mediastinal mass that is usually situated in a subcarinal or right paratracheal location. In other patients, the fibrosis invades or compresses adjacent structures and at times extends diffusely through the mediastinum. The latter condition, called fibrosing mediastinitis, is the most common nonmalignant cause of superior vena cava syndrome.91 Depending on which structures the fibrosis impinges, patients may also exhibit bronchial obstruction, pulmonary arterial or venous obstruction, or esophageal obstruction.91
In some cases of fibrosing mediastinitis, histologic examination of a surgical or postmortem specimen reveals only dense fibrosis. In other cases, a small caseous focus of infection can be identified, almost always caused by infection with Histoplasma capsulatum.91 The presumed mechanism in all cases is rupture of caseous material into the mediastinum, leading to an intense inflammatory reaction that heals with fibrosis.
Even when viable fungal organisms can be identified, the therapeutic response to amphotericin B is poor, and cortico-steroids are generally ineffective as well. Some studies have suggested that ketoconazole may be helpful.92 In some patients, surgical extirpation of the fibrotic mass is possible. Endovascular balloon angioplasty and placement of stents have been successful in the management of superior vena cava and pulmonary artery obstructions. In general, progression of the fibrosis is slow, and long-term survival is possible.
Anterior Mediastinal Masses
In addition to lymphomas, common causes of masses found in the anterior mediastinum are thymomas, teratomas, dermoid cysts, and retrosternal goiters.
Thymomas Thymomas may be found in adults of any age. They may arise from epithelial or lymphocytic cell lines. Most thymomas are benign, but some behave as malignant lesions and exhibit local invasion into adjacent structures. Reports of distant metastases are rare. Calcification may be present at the perimeter of or throughout the thymoma, but its presence does not necessarily signify that the lesion is benign. As many as one quarter to one third of patients with thymomas have myasthenia gravis, and some of these patients will experience a remission of symptoms after removal of the tumor. Other rare paraneoplastic syndromes that are associated with thymomas include red cell aplasia, Cushing syndrome, Graves disease, carcinoid syndrome, and hypogammaglobulinemia. Because of their malignant potential, suspected thymomas should be surgically excised in most cases. Other thymic lesions include thymic carcinoma, thymic carcinoid, thymolipoma, and thymic cysts.
Dermoid cysts and teratomas Germ cell tumors are most common in young adults 18 to 25 years of age. They originate from germ cell rests that were deposited in the mediastinum during embryogenesis. Dermoid cysts are usually benign cystic tumors composed of epidermal and dermal tissue; on occasion, hair, bone, or teeth form within these lesions. Teratomas consist of cells from all three embryonic origins (i.e., ectodermal, meso-dermal, and endodermal) and may be cystic (usually benign) or solid (usually malignant). As with thymomas, calcification may be present along the periphery of the tumor. A potential complication of dermoid cysts and cystic teratomas is rupture and subsequent discharge of the cyst contents into the mediastinum or tracheobronchial tree. Surgical excision is the treatment of choice. Other germ cell tumors of the mediastinum include seminoma and nonseminomatous malignant tumors.
Goiters Cervical goiters may extend inferiorly into the tho-rax.93 On occasion, masses within the mediastinum, usually located anteriorly, that have no palpable cervical connection prove to be intrathoracic goiters. These lesions are usually nodular colloid goiters that arise from the lower pole or isthmus of the thyroid gland and extend down into the chest; occasionally, such lesions prove to be adenomas or malignant tumors.
Most patients with nodular colloid goiters are asymptomatic, but some lesions grow sufficiently large to compress the trachea, causing dyspnea and stridor. Dysphagia, vascular compression, and vocal cord paresis or paralysis are other potential presenting manifestations. Uptake of radiolabeled iodine by a mediastinal mass on thyroid scan is diagnostic, but this is a relatively infrequent finding because most of these lesions are nonfunctioning goiters. Certain characteristic features of the CT image, including anatomic continuity with the cervical thyroid gland and particular patterns of calcification, have proved useful in identifying intrathoracic goiters. Patients with small, asymptomatic intrathoracic goiters can be observed or possibly given suppressive thyroid therapy; however, symptomatic patients require thyroidectomy, which can usually be achieved with a suprasternal incision.
Middle mediastinal masses
Mediastinal bronchogenic cysts usually originate near the main carina of the tracheobronchial tree, but they may extend into any of the three mediastinal compartments.88 They are lined with respiratory epithelium and contain a milky-white or brown mucoid material. Direct communication with the tracheo-bronchial tree is rare, although the potential exists for infection within the cyst and subsequent rupture into the airways or mediastinum. Bronchogenic cysts are usually discovered during childhood or early adulthood; in the latter age group, related symptoms are uncommon. Surgical excision is warranted for infected bronchogenic cysts or for cysts communicating with the tracheobronchial tree.
Posterior mediastinal masses
Neurogenic tumors are the most common primary neoplasms of the posterior mediastinum.88 They are round or oval masses in the paravertebral sulcus. Most patients are asymptomatic, although in some patients, chest or back pain or symptoms of bronchial compression develop. Tumors that arise from the nerve sheath (schwannomas) are usually benign; tumors derived from nerve cells may be benign (ganglioneuromas) or malignant (neuroblastomas or ganglioneuroblastomas). Neurofi-bromas derive from all nerve elements, including axons, sheath cells, and connective tissue; most are benign. Mediastinal neu-rofibromas may represent an isolated finding or may be part of generalized neurofibromatosis (von Recklinghausen disease). A rare tumor arising from paraganglionic cells is a mediastinal pheochromocytoma, which may be hormonally active.
Even benign neurogenic tumors may erode into an adjacent rib or cause pleural effusion. Some neurogenic tumors, most commonly neurofibromas, may extend into the spinal canal, causing widening of the intervertebral foramen; as a result, such tumors may assume a dumbbell shape. CT and MRI have for the most part supplanted myelography in the assessment of spinal extension of these so-called dumbbell tumors, which often require a coordinated thoracic and neurosurgical approach for resection.
Pneumomediastinum
Gas outside of normal GI structures may enter the mediastinum by several routes. First, it may collect in the mediastinum after esophageal or tracheobronchial rupture. Second, it may dissect into the mediastinum along fascial planes from the neck or oropharynx above (e.g., after dental extraction or tracheotomy) or from the retroperitoneum below (e.g., after colonic rupture or duodenal perforation). Third, after alveolar rupture, gas may track along the perivascular interstitium in the lung and enter the mediastinum at the pulmonary hilum. This third pathway is the likely route of gas entry in patients subjected to barotrauma from mechanical positive pressure ventilation or in those who suffer pneumomediastinum as a complication of bronchoscopy. Pneumomediastinum occurring in the setting of mechanical ventilation may be associated with the more serious complications of barotrauma, which include pneumothorax, gas embolization, and pneumopericardium. Most often, however, pneumomediastinum occurs independently of traumatic or iatrogenic causes, in which case it is referred to as spontaneous pneumomediastinum.
Etiology
Spontaneous pneumomediastinum occasionally occurs as a complication of pneumonia or asthma, but most often, it is found in young and otherwise healthy individuals.94 Symptoms are typically abrupt in onset and usually follow exaggerated respiratory efforts. Presumably, distended marginal alveoli rupture into the interstitium when the transpulmonary pressure is high; the elevated transpulmonary pressure would occur, for example, when a person inhales deeply or when a scuba diver holds his or her breath during ascent from an underwater depth. Thus, events that commonly precede spontaneous pneumomediastinum include parturition, cough, emesis, and straining at stool. In addition, numerous reports have appeared describing spontaneous pneumomediastinum after cocaine or marijuana use.
Diagnosis
Typical symptoms are retrosternal chest pain and dyspnea. The chest pain mimics that of pericarditis in that it is improved by sitting up and leaning forward; coughing, swallowing, and deep inspiration generally aggravate the pain.94 Because the free mediastinal gas often escapes cephalad into the subcutaneous tissues of the neck and supraclavicular area, patients may also complain of neck pain and sore throat, dysphagia, or a peculiar swelling and crepitation in the upper chest and neck. Physical findings may include a crunchlike sound heard in synchrony with the heartbeat (Hamman mediastinal crunch), diminished dullness on percussion of the heart, and subcutaneous emphysema. The last finding, which is detected by palpation of crepitations resulting from the formation of gas bubbles just below the surface of the skin, may in extreme cases of pneumomedi-astinum extend as far down as the arms, abdominal wall, and genitals. Gas in the mediastinum may also decompress into the pleural space, leading to a concomitant pneumothorax, usually on the left side.
The diagnosis is established with chest radiography.95 On pos-teroanterior view, a lucent zone of up to a few centimeters in width separates the cardiac silhouette from the medial border of the parietal and visceral pleurae. Gas may be seen outlining the aortic knob and extending in linear streaks up into the neck. On lateral view, gas collects between the sternum and the anterior border of the heart and outlines the aorta and other mediastinal soft tissue structures. In more subtle cases, lateral views of the neck reveal gas in the pretracheal fascia. In mild cases, CT may be necessary to detect the air in the mediastinum.96
Treatment
Spontaneous pneumomediastinum in spontaneously breathing adults resolves without specific therapy within a few days, and recurrences are uncommon. In contrast to spontaneous pneumothoraces, spontaneous pneumomediastinum is not recurrent. Thus, other than treating any underlying lung disease, management consists of reassurance, avoidance of strenuous activities, and use of analgesics if needed. Breathing-enhanced concentrations of oxygen may accelerate the rate of gas resorption. In adults, it is exceedingly rare that gas within the mediastinum is of sufficient pressure to compress vascular structures; therefore, surgical decompression of the mediastinal space by techniques such as tracheotomy is not indicated.