Pleural thickening
Pleural thickening develops when pleural inflammation of any cause heals with the formation of fibrous tissue involving the visceral or parietal pleural surfaces. The costophrenic angle is most frequently involved, causing blunting of the normal recess. Localized pleural thickening over the apex of the lung—the so-called apical pleural cap—usually involves fibrous scarring of the apical lung merging into the adjacent visceral pleura. Although apical pleural thickening was once thought to be indicative of granulomatous disease from tuberculosis, more recent studies have documented the absence of any correlation with tuberculosis. The etiology of this asymptomatic finding is unknown.
Pleural thickening may be bilateral or unilateral. Extensive bilateral pleural thickening, extending along the lateral margins of the chest and even to the apex of the lung, is frequently the result of exposure to inorganic fibers—most often asbestos and, occasionally, talc. Other causes of bilateral pleural thickening include uremia and collagen vascular diseases, especially SLE and rheumatoid arthritis. Diffuse unilateral pleural thickening usually results from one of three causes: hemothorax, bacterial empyema, or tuberculous pleurisy.
Diffuse pleural thickening may be sufficiently dense to entrap the underlying lung, preventing its full expansion and impairing gas exchange [see Figure 8].65 This condition is referred to as fi-brothorax. During the months immediately after hemothorax or empyema, inflammatory pleural thickening may gradually re-sorb. However, once an organized fibrous peel has formed, surgical decortication is the only available therapy. If the underlying lung tissue is relatively normal, resection of a thick pleural peel may result in significant improvement in ventilatory function.
In rare cases, localized or diffuse pleural thickening can entrap a small region of lung, producing a masslike lesion termed rounded atelectasis. It can be difficult to differentiate such lesions from other, more serious mass lesions (e.g., neoplasm).
Pleural calcification
Diffuse, sheetlike calcification may develop in patients with hemothorax, bacterial empyema, or tuberculous pleurisy. Unlike the pattern of calcification that is observed in asbestos-related pleural plaques, calcium deposition after these conditions always takes place along the visceral pleura, thereby outlining the inner margin of the pleural thickening. In addition, calcification is generally found at the level of the midthorax, sparing the diaphragmatic pleural surface.
Pneumothorax
Etiology
Pneumothorax, the presence of gas within the pleural space, indicates that disruption of the visceral or parietal pleura has oc-curred.67 The gas that enters the pleural space may come from various sources. For example, in a penetrating injury involving the chest wall and parietal pleura, the gas enters from the outside environment. Alternatively, the gas may come from a gas-filled gastrointestinal structure; this situation might arise as a result of a ruptured esophagus, or it could arise from a ruptured intra-abdominal viscus and subsequent escape of gas across the diaphragm from a pneumoperitoneum. Most commonly, the source of the gas is the lung—after alveolar or tracheobronchial injury, after blunt or penetrating trauma, or as a complication of invasive diagnostic and therapeutic procedures, such as thora-centesis, attempted percutaneous cannulation of a central vein, acupuncture, or intercostal nerve block.
Pneumothorax caused by disruption of the visceral pleura may also result from focal pulmonary processes. Focal destructive processes may involve the visceral pleura as a primary site or may extend to the pleura from adjacent lung tissue. Examples of focal pulmonary processes that cause pneumothorax include bronchogenic carcinoma, rheumatoid lung nodule, thoracic en-dometriosis,45 necrotizing pneumonia, and pulmonary infarct. In the past, tuberculosis was frequently implicated as a cause of pneumothorax. Pneumocystis carinii pneumonia, a complication of AIDS, has become a common cause of pneumothorax, particularly in patients receiving aerosolized pentamidine, those who smoke cigarettes, and those with pneumatoceles seen on chest radiograph or CT scan.68
Diffuse diseases of the lung parenchyma can also cause pneu-mothorax. Such diseases can greatly distort the lung architecture, resulting in an uneven distribution of ventilation within the lung. In this setting, localized alveolar overdistention, along with weakened alveolar walls, leads to an increased incidence of associated pneumothorax. The most common of these diffuse processes are the obstructive lung diseases, specifically emphysema, asthma, and cystic fibrosis.69 Rarer conditions that also carry an increased risk of pneumothorax include pulmonary lymphan-giomyomatosis,50 tuberous sclerosis, eosinophilic granuloma, scleroderma, and congenital disorders of connective tissue. In some cases, the visceral pleura remains intact but alveolar gas gains entry to the pleural space via the mediastinum. When perivascular alveoli rupture, alveolar gas can dissect centripetally along the bronchovascular interstitium to the mediastinum, entering the pleural space through the mediastinal parietal pleura.
Classification of pneumothorax
A commonly used classification of pneumothorax recognizes traumatic pneumothorax and iatrogenic pneumothorax as distinct clinical entities and lumps all other causes of pneumothorax under the somewhat misleading label of spontaneous pneu-mothorax. When spontaneous pneumothorax occurs in patients with underlying pleural or parenchymal disease, it is called secondary spontaneous pneumothorax. When no underlying lung disease is evident, it is called idiopathic spontaneous pneumo-thorax. Although patients with idiopathic spontaneous pneumo-thorax are otherwise healthy, most have subpleural apical blebs, frequently associated with more diffuse centrilobular emphysema detectable by CT scan70; during surgery, the blebs are often found to have ruptured into the pleural space. These abnormalities may have a genetic etiology.71 Secondary spontaneous pneu-mothorax is a more serious problem than idiopathic spontaneous pneumothorax because patients with secondary spontaneous pneumothorax typically have impaired lung function.
Epidemiology
Secondary Spontaneous Pneumothorax
The incidence of secondary spontaneous pneumothorax depends on the underlying disease process. The incidence of pneu-mothorax in patients with chronic obstructive lung disease is approximately 26 per 100,000 per year, and the incidence is directly related to the severity of obstruction.67 Pneumothorax will develop in 5% to 8% of cystic fibrosis patients at some point in their lifetime; however, it occurs in 16% to 20% of cystic fibrosis patients older than 18 years. Pneumothorax occurs in 2% to 6% of HIV patients and is almost always associated with P. carinii pneumonia.67 Twenty-five percent of patients with eosinophilic granuloma and 80% of patients with pulmonary lymphangio-myomatosis have pneumothorax at some point in their disease course, and pneumothorax can be a presenting manifestation in both diseases.
For most underlying lung diseases, the rate of recurrent pneu-mothorax is similar to that of idiopathic spontaneous pneumo-thorax (39% to 47%),67 although patients with cystic fibrosis have a much higher recurrence rate (68% to 90%).69
Idiopathic Spontaneous Pneumothorax
Idiopathic spontaneous pneumothorax has an incidence of approximately 4.3 cases per 100,000 patient-years. The peak incidence is in persons between 20 and 30 years of age, and the male-to-female ratio is approximately 5:1. Patients often have a tall, thin stature72 and very frequently are cigarette smokers. The precise mechanism whereby male sex, asthenic habitus, and cigarette smoking predispose to apical pleural bleb formation or rupture is unknown. One study detected anomalies of the bronchial tree in the majority of patients with spontaneous pneu-mothorax, suggesting associated congenital abnormalities of lung structure.73 How these anomalies may relate to the occurrence of pneumothorax is unclear. Common misconceptions are that strenuous physical activity is frequently a trigger for the development of pneumothorax and that patients are at increased risk during airplane travel. In fact, most studies have found that the onset of symptoms of pneumothorax usually occurs at rest or during light activity, and a study of pneumothoraces among pilots in the United States Air Force found that very few episodes occurred during flight.74
Diagnosis
The most common symptoms of pneumothorax are chest pain and dyspnea. The pain may be a dramatic, severe, stabbing unilateral chest pain with a sudden, explosive onset, sometimes radiating to the ipsilateral shoulder or scapular area. In other cases, the discomfort may be more modest and more easily tolerated. Often, patients will recall previous transient episodes of pain that were similar to, although milder in degree or shorter in duration than, the one that finally caused them to seek medical attention. Dyspnea develops in most patients. The dyspnea is more severe when the pneumothorax is large and when there is significant underlying lung disease (i.e., secondary spontaneous pneumothorax).
Secondary Spontaneous Pneumothorax
In a patient with secondary spontaneous pneumothorax associated with underlying emphysema, the diagnosis is particularly difficult to make on the basis of physical findings. Decreased lung elastic recoil and residual hyperinflation keep the lung from fully collapsing and limit changes in the size of the thoracic cage. In addition, in patients with emphysema, physical findings such as hyperresonance and diminished breath sounds may be found over the contralateral lung. In a patient with chronic airflow obstruction, the sudden onset of chest pain and worsened dyspnea should raise the suspicion of pneumothorax. Confirmation of the diagnosis of pneumothorax usually requires a chest radiograph.
Idiopathic Spontaneous Pneumothorax
Characteristic physical findings on the involved side include expansion of the hemithorax (caused by release of the ipsilateral chest wall from the recoil forces of the lung), hyperresonance, diminished fremitus, diminished transmission of voice sounds, and distant or absent breath sounds.
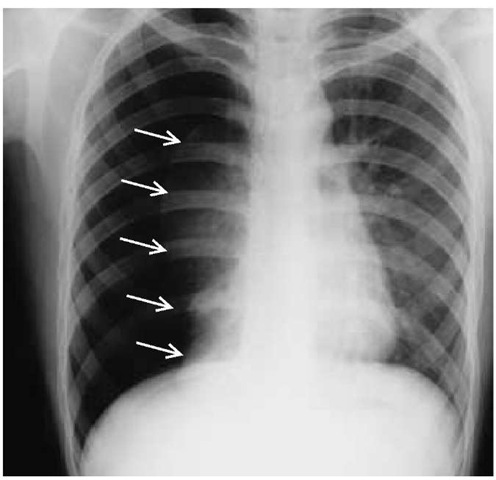
As gas collects in the pleural space, the lung recoils from the chest wall toward the hilum. The presence of a pneumothorax can be identified on a chest radiograph by visualization of a thin (=1 mm) linear shadow made by the visceral pleura as it passes along a plane tangential to the x-ray beam. This linear shadow, marking the outer rim of the lung, follows the contour of the inner aspect of the chest wall, and no lung markings (i.e., bron-chovascular shadows) can be seen peripheral to it. In cases in which the pneumothorax is small and gas collects over the apex of the lung, it may be difficult to distinguish the visceral pleural line from superimposed rib margins. When the chest radiograph is taken with the patient in the supine position, a pneumothorax will collect along the costophrenic sulcus rather than along the apex of the lung. This so-called deep sulcus sign may be useful in identifying occult pneumothorax in hypotensive patients in whom upright chest radiographs are inadvisable. Chest radiographs taken during full expiration have not been shown to enhance the detection of pneumothorax.75 Pitfalls in the radi-ographic diagnosis of pneumothorax include confusion regarding two other causes of curvilinear shadows in the chest: (1) extrathoracic skin folds and (2) intrapulmonic cysts or bullae.
Figure 9 This chest radiograph of a patient with pneumothorax demonstrates virtually complete collapse of the right lung. There has been a slight shift of the mediastinum toward the contralateral side. The visceral pleura (arrows) can be clearly identified because gas is present on both sides.
It is difficult to accurately estimate the size of a pneumothorax relative to the size of the hemithorax by casual inspection of the chest radiograph. As a rough indicator, a collection of gas around the lung that has an average thickness of 1 in. represents a 30% pneumothorax. When collapse of the lung is complete (100% pneumothorax), the lung forms a fist-sized opacity near the hilum, and the mediastinum may shift slightly toward the contralateral lung; the diagnosis is obvious on x-ray [see Figure 9].
A small pleural effusion is commonly present in pneumothorax. It is detected on radiograph by blunting of the costophrenic sulcus. The pleural liquid is usually bloody. The effusion is probably formed as a result of the rupture of small blood vessels within pleural adhesions.
Treatment
Secondary Spontaneous Pneumothorax
Because initial or recurrent episodes of secondary spontaneous pneumothorax can be life threatening, aggressive treatment is required.67,76 Patients should be hospitalized and a chest tube placed for drainage; observation and simple aspiration are not adequate treatment. The methods for preventing recurrence of secondary spontaneous pneumothorax are the same as those of idiopathic spontaneous pneumothorax (e.g., thoracotomy, video-assisted thoracotomy, pleurodesis) (see below), although many experts feel that application of such methods should be utilized on the first occurrence of secondary spontaneous pneumothorax.
Idiopathic Spontaneous Pneumothorax
Treatment of idiopathic spontaneous pneumothorax is directed in part at allowing the collapsed lung to expand fully again and in part at preventing recurrences.67 In mildly symptomatic patients with moderate pneumothorax, simple aspiration of the pneumothorax may be successful in 60% to 70% of cases.77 A patient with a small (15% to 20%) pneumothorax who is asymptomatic or minimally symptomatic can be safely observed, and the pneumothorax can be allowed to resorb spontaneously. Complete resolution of a pneumothorax usually requires approximately 10 days, provided there is no further air leak. Resolution can be accelerated by the administration of supplemental oxygen. Supplemental oxygen lowers the nitrogen content of blood, thereby increasing the pressure gradient for nitrogen that favors transfer of gas from the pleural space into the venous end of the pleural capillaries. Strict bed rest does not hasten resolution and is not necessary. A large pneumothorax in a symptomatic patient should be evacuated promptly with an intercostal smallbore (14 French) or large-bore (28 French or greater) chest tube passed cephalad into the apex of the chest. The air leak from the lung may seal immediately or may persist for 3 to 5 days until the tear in an apical bleb heals. An air leak from the lung that persists for more than 7 days is considered by many physicians to be an indication for surgical intervention; at thoracoscopy or thoracotomy, the blebs can be oversewn or excised by wedge resection and the pleura abraded or pleurodesis performed.
Incidence of recurrence Idiopathic spontaneous pneumo-thorax often recurs. At least 20% to 30% of patients with idiopath-ic spontaneous pneumothorax will experience an ipsilateral recurrent pneumothorax within the ensuing 5 years; most recurrences occur within a year after the initial event. According to some reports, the rate of initial recurrence may be as high as 50%. Recurrences are more common in women and taller men and are reduced by smoking cessation.78 Ninety percent or more of recurrences are ipsilateral, despite the fact that the underlying abnormality (i.e., apical subpleural blebs) is bilateral in more than half the cases.79 Simultaneous bilateral idiopathic spontaneous pneu-mothoraces are fortunately infrequent, occurring in approximately 1% of cases; surprisingly, when they do occur, they are rarely fatal. After the first ipsilateral recurrence of a pneumothorax, subsequent recurrences become increasingly likely.
Management of recurrence There is debate regarding the best method of preventing recurrences and the optimal timing for such an intervention.76 Because half or more of patients with idiopathic spontaneous pneumothorax will never suffer a recurrence, it seems reasonable to withhold preventive treatment until after a recurrence. At that point, the probability of further recurrences is quite high, and the discomfort and inconvenience associated with recurrent pneumothoraces would have become increasingly apparent to the patient. Traditional and definitive treatment for prevention of recurrences involves thoracotomy: apical blebs are oversewn or excised by wedge resection, and adhesions are induced between the lung and chest wall by abrasion of the pleural surface with dry gauze or by partial parietal pleurectomy. Recurrent pneumothorax occurs after this procedure in 0% to 2% of patients. Video-assisted thoracoscopy has allowed these procedures to be performed without thoracotomy, resulting in less pain and shorter hospital stays.80 Attempts have also been made to achieve pleurodesis without thoracotomy via a chest tube. This technique is the same as that used for the management of pleural effusions in patients with malignant disease [see Hydrothorax Caused by Malignant Disease, above]. Recurrence of spontaneous pneumothorax is reduced after doxycy-cline administration or talc pleurodesis.
Tension pneumothorax
Occasionally, gas enters the pleural space during the inspira-tory phase and is prevented from escaping during expiration, presumably because an airway or tissue flap acts as a one-way valve. Under these circumstances, there is a progressive increase in the amount of pleural gas, and the pleural gas is under increased pressure (i.e., tension). This situation is referred to as tension pneumothorax. Tension pneumothorax occurs in only 1% to 2% of the cases of idiopathic spontaneous pneumothorax. However, it is a more common manifestation of the barotrauma that may occur as a complication of positive pressure mechanical ventilation.
Diagnosis
Tension pneumothorax is a medical emergency. Patients are often dyspneic at rest and gasping for breath. Cyanosis and hypotension are common. The diagnosis cannot be established by a plain chest radiograph, although a marked contralateral shift of the mediastinum and depression or inversion of the ipsilateral hemidiaphragm are suggestive.
Treatment
If there is acute distress, immediate action must be taken to remove gas from the pleural space. Introduction of a small-bore plastic catheter together with a needle through an intercostal space may suffice for emergency relief if delay in placing a full-sized chest tube is anticipated. In patients with tension pneu-mothorax, the gas that is under pressure will rush out of the chest through the open catheter. If a Heimlich flutter valve, which allows one-way passage of gas, is attached to the catheter, a series of coughs or Valsalva maneuvers will allow almost complete evacuation of the remainder of the pneumothorax that is not under tension. Because of the life-threatening nature of tension pneumothorax, a procedure to prevent recurrence should be undertaken after the first such event in cases involving idiopathic spontaneous pneumothorax.