The chest wall consists of the parietal pleura, rib cage, and muscles. The abdominal contents and abdominal wall function as part of the rib cage in that they influence the resting position and movement of the diaphragm.
The respiratory pump apparatus is composed of the rib cage and its musculature, including the intercostal muscles, diaphragm, and accessory muscles of respiration.1 Optimal pumping action requires structural integrity and the synchronized contraction of the intercostal muscles and diaphragm. Respiratory pump function may be impaired by mass loading from obesity, skeletal abnormalities, neuromuscular disorders, or restriction of lung movement from pleural disease. Cervical strap or abdominal muscles may be recruited for assistance when respiratory pump function is impaired. Because the diaphragm is the major power generator for the respiratory pump, loss of rib cage function alone may be insufficient to cause ventilatory failure; ankylosis and paralysis of the rib cage are often associated with a normal resting carbon dioxide tension (Pco2).
Respiratory Pump Dysfunction
Respiratory pump dysfunction may vary from trivial to severe. Severe dysfunction restricts lung expansion and may cause dyspnea and hypercapnic ventilatory failure because of the small tidal volumes that increase the proportion of wasted ventilation per breath, despite a compensatory increase in the rate of respiration [see Table 1 ]. The result is a decrease in alveolar ventilation with hypercapnia and hypoxemia. Hypercapnia is often most severe during sleep because of a decrease in ventilatory drive and a sleep-associated increase in upper airway resistance. Hypercapnia and the associated hypoxia may in turn cause vasoconstrictive pulmonary hypertension and cor pulmonale [see 14:XI Pulmonary Hypertension, Cor Pulmonale, and Primary Pulmonary Vascular Diseases]. Weakness of expiratory muscles from neuromuscular disease may produce ineffective cough and result in recurring atelectasis or infections. Severe respiratory pump dysfunction differs from alveolar and interstitial lung disease, in which ventilatory abnormalities result from alterations in the lung parenchyma [see Table 2].
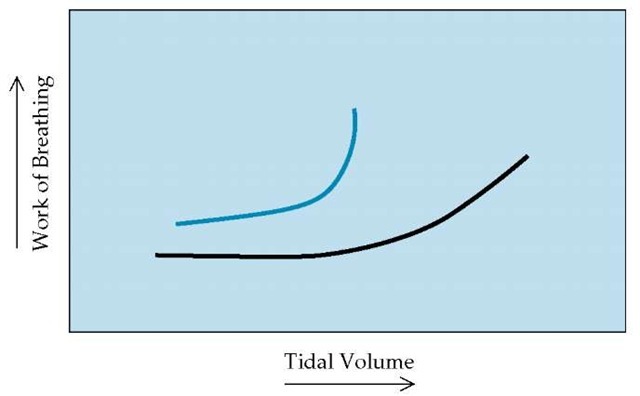
When the respiratory pump is impaired, ventilation will be determined by (1) the efficiency of the inspiratory muscles, (2) the strength of the inspiratory muscles, and (3) the impedance to the pumping action of these muscles. The efficiency of the respiratory muscles is determined by their length and by the resulting mechanical action on the pump apparatus. Shortening of the inspiratory muscles from hyperinflation or chest wall deformity reduces their pumping efficiency. Paralysis of either the chest wall or the diaphragm produces an observable paradoxical movement of the paralyzed component during inspiration; this movement results in inefficiency of the pump apparatus. The strength of the inspiratory muscles may be reduced by neuromuscular disease or metabolic disturbances, such as hy-pokalemia or hypophosphatemia. Ventilatory ability is proportional to the remaining respiratory muscle strength but may be disproportionately reduced if there are concomitant mechanical problems of the respiratory system. Finally, either increased airway resistance or decreased respiratory system compliance may impede the pumping action of the inspiratory muscles and reduce ventilation. Respiratory system compliance may be reduced by morbid obesity, chest wall deformity, circumferential pleural disease, or parenchymal disease. Patients with a poorly compliant respiratory system must exert more effort than healthy patients to achieve equivalent tidal volumes [see Figure 1], so they take smaller breaths to minimize respiratory muscle fatigue but must compensate by increasing their breathing rate.
Obesity and Its Impact on Respiratory Function
Obesity imposes a restrictive load on the thoracic cage, both directly because weight has been added to the rib cage and indirectly because of the large abdominal panniculus, which impedes the motion of the diaphragm when the person is supine.2,3 In addition, obese patients, particularly males, may experience increased respiratory resistance and resultant airflow limitation that may be related to breathing at lower lung volumes, increases in pulmonary blood volume, or both.
Obesity characteristically causes a decrease in functional residual capacity that becomes significant only in the presence of coexisting conditions such as obstructive lung disease, in which airway closure occurs at lower lung volume, leading to hypoxemia.
Obesity in otherwise healthy patients causes little interference with lung function at rest. Generally, vital capacity and total lung capacity remain normal except in the most severe instances of morbid obesity.
Table 1 Relations among Tidal Volume, Respiratory Frequency, and Arterial and Alveolar Oxygen Tension
|
Volume (L) |
 |
 |
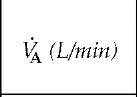 |
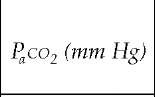 |
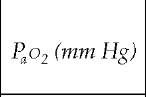 |
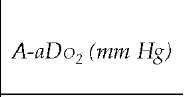 |
|||
|
Normal respiratory function |
0.50 |
0.35 |
0.15 |
30% |
12/min |
4.20 |
40 |
90 |
10 |
|
Restrictive disorder |
0.25 |
0.10 |
0.15 |
60% |
30/min |
3.00 |
56 |
70 |
10* |
Note: this table demonstrates how restrictive disorders of the respiratory system resulting from neuromuscular or chest wall disease may produce hypercapnia and hypoxemia by decreasing the amount of ventilation per breath.
*The elevated Paco2 has resulted in arterial and alveolar hypoxia. However, in the absence of atelectasis or another concomitant disease that would increase the ventilation-perfusion mismatch, the A-aDo2 remains normal.
A-aDo2—alveolar-arterial difference in oxygen f—respiratory frequency Paco2—arterial carbon dioxide tension Pao2—arterial oxygen tension Va—alveolar portion of the tidal volume Va—alveolar ventilation (f x Va) Vd—dead space portion of the tidal volume Vd/Vt—ratio of functional dead space volume to tidal volume, or the wasted ventilation ratio Vt—tidal volume
Table 2 Respiratory Parameters in Kyphoscoliosis, Neuromuscular Syndrome, and Diffuse Parenchymal Lung Disease
In patients with impaired ventilatory drive, the mechanical work load imposed by obesity may not be countered by increased respiratory effort. Under such circumstances, chronic daytime hypercapnia may develop, most commonly in the setting of obstructive sleep apnea but also in the absence of sleep-disordered breathing. The pathogenetic role of obesity in obstructive sleep apnea [see 14:VI Ventilatory Control during Wakefulness and Sleep and 11:X1II Disorders of Sleep] may relate in part to fatty encroachment on the upper airways.
Obese patients may experience significant dyspnea during exercise because of the increased work required to move the heavy chest and abdomen and because of overall poor conditioning. The tachypneic shallow breathing pattern during exercise in morbidly obese patients reflects the combined effects of this mass loading and diminished compliance of the respiratory system [see Figure 1].
Weight loss is the most important therapy for patients with respiratory problems related to obesity. For patients with associated sleep disorders of breathing, appropriate treatment of obesity may alleviate hypercapnia [see 14:VI Ventilatory Control during Wakefulness and Sleep and 11:XIII Disorders of Sleep]. Noninva-sive ventilation may help decrease the symptoms of daytime hypercapnia.6
Skeletal Abnormalities that Affect Respiratory Function
Deformities of the costovertebral skeletal structures may affect compliance of the thoracic cage, its shape and volume, and, ultimately, pulmonary compliance. Effects vary from unde-tectable to severe.
kyphoscoliosis
The two basic types of costovertebral skeletal deformity—sco-liosis, a lateral curvature with rotation of the vertebral column, and kyphosis, an anterior flexion of the spine—are usually found in combination. Approximately 80% of cases of kyphoscoliosis are idiopathic. Idiopathic kyphoscoliosis commonly begins in late childhood or early adolescence and may progress in severity during these years of rapid skeletal growth. The incidence of kyphoscoliosis in females is four times higher than that in males. The remaining 20% of cases of kyphoscoliosis are found in association with neuromuscular disorders (e.g., sy-ringomyelia, neurofibromatosis, or poliomyelitis), congenital vertebral defects (e.g., hemivertebrae), acquired vertebral abnormalities (e.g., tuberculous spondylitis or osteomalacia), or deforming chest wall processes (e.g., sequelae of empyema or as a result of thoracoplasty).
Respiratory Compromise
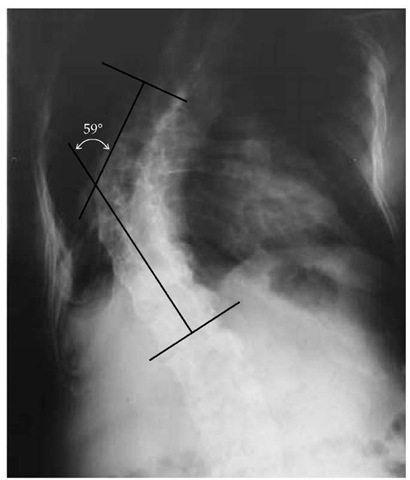
Of the various chest deformities that produce ventilatory failure, kyphoscoliosis is the most common. The degree to which ventilation is reduced is determined by the severity of deformity and the degree of neuromuscular weakness. A standard technique for measuring the degree of spinal curvature gives an indication of the potential risk for respiratory compromise [see Figure 2]. Mild to moderate deformities (scoliotic angle < 60°) are associated with minimal to mild restrictive ventilatory defects.
Figure 1 When the respiratory pump is encumbered by structural changes such as obesity, kyphoscoliosis, or ankylosing spondylitis (blue line), the work of breathing at rest is higher than it is for a healthy person (black line). The work of breathing required to maintain the tidal volumes needed during exercise may be prohibitive for patients with these disorders, forcing them to adopt a shallow tachypneic breathing pattern in response to an increased ventilatory requirement.
Figure 2 In this radiograph of the spine in a patient with kyphoscoliosis, straight lines are passed through the upper and lower limbs of the curvature. The angle inscribed by these two lines defines the scoliotic angle.
Dyspnea may occur during exercise and is most often caused by deconditioning and lack of regular aerobic exercise rather than by any alteration of lung function.7 As the scoliotic curvature worsens, vital capacity and total lung capacity decline significantly, and dyspnea on mild or moderate exertion becomes a common complaint. Kyphoscoliosis may distort the respiratory pump, so that inspiratory power becomes limited even in the absence of a neuromuscular disease, such as poliomyelitis. The severity of hypercapnia is therefore related to both the severity of deformity and the degree of inspiratory muscle weakness.
Severe deformities (scoliotic angle > 100°) can be associated with prominently restricted lung volumes; typically, total lung capacity is reduced to 50% or less of the predicted value. Such restriction may lead to chronic alveolar hypoventilation, hypox-emia, pulmonary hypertension, and, ultimately, cor pulmonale.8 Long-term follow-up of patients with kyphoscoliosis suggests that those with a vital capacity less than 45% of the predicted value and a scoliotic angle greater than 110° are at the greatest risk for respiratory failure.9 Kyphoscoliosis of such severity may cause compression of underlying lung tissue, thereby elevating the alveolar-arterial difference in oxygen (A-aDO2). In most cases of kyphoscoliosis, however, the A-aDO2 remains at normal or near-normal levels, and significant hypoxemia is present only when hypercapnia develops. These findings contrast with restrictive ventilatory disorders caused by diffuse parenchymal lung disease, in which the A-aDO2 is characteristically elevated and hypoxemia is often associated with hypocapnia until late in the course of disease.
Treatment
Acute complications Patients with severe kyphoscoliosis may live for many years without succumbing to respiratory insufficiency. Such patients, however, are vulnerable to any respiratory tract infection or central nervous system depressant. Because breathing is chronically restricted, increased central neural output and physical work are required to maintain ventilation; relatively minor insults, such as bacterial or viral bronchitis or pneumonia, may represent an increment in load sufficient to produce frank respiratory failure. In addition, standard doses of narcotics or sedatives may suppress chronically hyperactive control mechanisms to a level sufficient to precipitate acute respiratory failure.
Thus, immunization with influenza and pneumococcal vaccines, early treatment of respiratory tract infections, and strict avoidance of CNS depressants are important in the management of kyphoscoliosis. Episodes of hypercapnic respiratory failure precipitated by reversible conditions respond well to short-term supportive measures, including bronchopulmonary drainage, mechanical ventilatory support, and oxygen supplementation.
Chronic complications Chronic respiratory insufficiency may ensue after several years. Older patients with kyphoscoliosis are at risk for respiratory failure because the angle of curvature typically worsens with age. Although most cases of idiopathic scoliosis stabilize just after puberty, further spinal deformity may result from the osteoporosis, vertebral body weakening, and loss of muscle tone that accompany older age. Surgical procedures to straighten and stabilize the vertebral column usually fail to restore ventilatory capacity. Such procedures are useful early in the course of kyphoscoliosis, when they may prevent progression of the deformity before respiratory compromise develops.10
Supportive measures may sustain meaningful life for many years, even in patients with chronic respiratory failure. Many can adapt very well to a state of chronically disordered gas exchange. Chronic hypoxemia accompanied by secondary eryth-rocytosis, worsening of pulmonary hypertension, and cor pul-monale should be treated with supplemental oxygen administration. Such therapy can be augmented by nocturnal ventilatory support.
Although kyphoscoliosis is not a primary sleep-related breathing disorder, the degree of oxygen desaturation that occurs during sleep is greater than that observed in other types of lung disease, possibly because the baseline hypoxemia and hypercapnia are more severe and lung volumes are smaller.12 Thus, nocturnal oxygen therapy and mechanical ventilatory support during sleep often improve functional status and symptoms. In fact, many patients who achieve normal levels of arterial carbon dioxide tension (PaCO2) during sleep by means of mechanical ventilators can sustain normal or near-normal arterial blood gas levels throughout the day. Devices used for mechanical ventilation in such patients include the iron lung, specially fitted thoracoabdominal negative pressure ventilators, and positive pressure ventilators applied via tracheostomy or nasal mask.