Albinism
Definition
Albinism is an uncommon, complex congenital disorder characterized by hypopigmentation of the hair, eyes, and skin. Albinism is generally subclassified as oculocutaneous albinism (OCA) and ocular albinism (OA); in the latter, reduction of melanin is limited to the eye.66-71 Sometimes, different mutations in the same gene can cause OCA or OA.
Epidemiology
OCA has been reported by investigators in all mammalian orders and in all human ethnic groups. It is one of the most widely distributed genetic abnormalities in the animal kingdom. Human albinism has been noted throughout history. OCA is the most common inherited disorder of generalized hypopigmentation.
Etiology and Pathogenesis
Albinism may result from primary defects that are specific for the melanin synthetic pathway or from defects that are not specific for melanin synthesis. Mutations in seven genes have been reported to cause OCA or OA.67,68 They include the tyrosinase gene (OCA1 on chromosome 12q1), the oculocutaneous albinism gene (OCA2, a missed mutation of the P gene on 15q11), the ty-rosinase-related protein 1 gene (OCA3), the HPS gene (Herman-sky-Pudlak syndrome at 10q23 and mutations of the |3A-adaptin gene), the CHS gene (Chediak-Higashi syndrome), and the OA1 gene (X-linked ocular albinism).
Diagnosis
Clinically, the most severe disease is observed in OCA1A, which is OCA resulting from mutations in the tyrosinase gene. It is characterized by absent tyrosinase activity, which results in complete absence of melanin in the eyes, skin, and hair. There is no improvement with age. Affected individuals have marked photophobia, nystagmus, and profound sun sensitivity because of the inability to tan.
OCA1B, or yellow albinism, is less severe. Tyrosinase activity is low or absent, and pigmentation of the hair and skin improves with age. In contrast to OCA1A, pigmented freckles and lentig-ines develop with age.
OCA1-MP, or minimal-pigment OCA, is characterized by white skin and hair at birth. Iris pigment is present at birth, or it appears during the first decade of life. All reported cases have been in white persons. The tyrosinase gene mutation produces a less active enzyme.
Temperature-sensitive OCA (OCA1-TS) is characterized by white skin and hair and blue eyes at birth and by development of patterned pigmentation by puberty. Darker hair develops in cooler areas (extremities), and white hair is retained in warmer areas (axilla and scalp). The pattern results from a tyrosinase mutation that causes a temperature-sensitive enzyme.
OCA2, tyrosinase-positive OCA with normal tyrosinase activity, is the most common variety. The hair darkens with age, but the skin remains white. Pigmented nevi, lentigines, and freckles develop and are especially pronounced in sun-exposed areas. This type has recently been ascribed to mutation of the P gene, which encodes the tyrosinase-transporting membrane protein. The P gene is on chromosome 15q.
OCA3 encompasses the Rufous variety and some cases of brown albinism. Clinically, there is minimal pigment reduction in the hair, eyes, and skin.
The secondary varieties of albinism in which the primary defect is not specific for the melanin synthetic pathway include Her-mansky-Pudlak syndrome,71 Chediak-Higashi syndrome, Cross-McKusick-Breen syndrome, Prader-Willi syndrome, and Angel-man syndrome.
The autosomal recessive Hermansky-Pudlak syndrome is characterized by low to absent tyrosinase activity. The HPS gene has been mapped to chromosome 10q23.71-73 Skin and hair color varies from white to light brown. Freckles and lentigines develop with age.
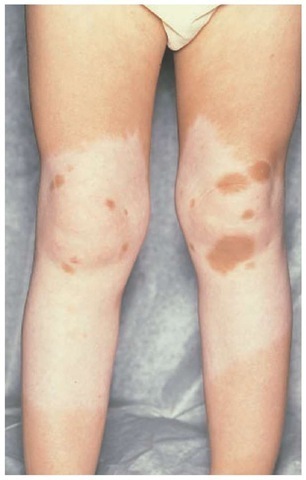
Figure 5 A patient with piebaldism has the classic midextremity areas of depigmentation with islands of hyperpigmentation.
Iris pigment correlates with hair and skin pigmentation. Affected individuals experience a hemorrhagic diathesis secondary to a platelet-storage-pool deficiency. Their platelets lack storage granules (i.e., sites of storage for serotonin, calcium, and adenine nucleotides). Ceroidlike deposits are present in macro-phages of the bone marrow, lungs, liver, spleen, and gastrointestinal tract. These patients bruise easily and are subject to epis-taxis and gingival bleeding. Pulmonary fibrosis and granuloma-tous colitis develop as a consequence of the ceroid deposits.
Chediak-Higashi syndrome consists of hypopigmentation, recurrent sinopulmonary bacterial infections, peripheral neuropathy, and giant lysosomal granules, with death occurring at an early age as a result of lymphoreticular malignancies. The CHS gene locus is on chromosome 1q29. Chediak-Higashi syndrome must be distinguished from Griscelli syndrome, which is characterized by partial albinism, lymphohistiocytosis, immunodeficiency, neutropenia, and thrombocytopenia. Griscelli syndrome has been mapped to chromosome15q21, around the myosin-Va gene. However, the presence of giant lysosomal granules is pathognomonic for Chediak-Higashi syndrome.
Cross-McKusick-Breen syndrome includes hypopigmenta-tion, microphthalmia, nystagmus, and severe mental and physical retardation.
Prader-Willi syndrome is a developmental syndrome characterized by mental retardation, neonatal hypotonia, and poor feeding, followed by hyperphagia and obesity later in life. Short stature, hypogonadism, and inappropriate emotional behavior constitute the syndrome. Fifty percent of patients have a deletion on the long arm of chromosome 15. Patients have ocular abnormalities and skin and hair hypopigmentation consistent with OCA.
Mutation of the P gene has been reported in Angelman syndrome and is also characterized by mental retardation, abnormal behavior, and hypopigmentation. The pattern of hypopigmenta-tion is similar to that in Prader-Willi syndrome. In addition, An-gelman syndrome is associated with a deletion on chromosome 15. However, in contrast to Prader-Willi syndrome, the deletion occurs on the maternal chromosome.
Treatment
The management of patients with albinism should include genetic counseling and patient education regarding the use of sunscreens and clothing for protection against ultraviolet radiation-induced damage. Magnifiers are beneficial for ocular symptoms.
Complications
The long-term consequences of albinism are solar keratoses and basal and squamous cell carcinomas. Malignant melanoma is uncommon.
Piebaldism
Definition
Piebaldism is a rare autosomal dominant congenital disorder of pigmentation. It is a stable leukoderma and is characterized by patches of white skin and white hair. The affected areas are principally the frontal scalp, forehead, ventral chest, abdomen, and extremities. A white forelock occurs in 80% to 90% of patients.
Epidemiology
Although rare, piebaldism occurs in all ethnic groups worldwide. Its estimated occurrence is one in 100,000 persons. It is found with equal frequency in males and females.
Etiology and Pathogenesis
Molecular genetics studies have shown that piebaldism results from mutations of the KIT proto-oncogene, which encodes the cell surface receptor tyrosine kinase for mast cell or stem cell factor located on chromosome segment 4q12. Mutations occur in the highly conserved tyrosinase domain of KIT. A number of different mutations in the KIT gene can cause piebaldism.76-80 The locations of the KIT gene mutation correlates with severity of disease. Mutations of the intracellular ty-rosine kinase domain are associated with the most severe phe-notypes.77 Reduced KIT function arrests the migration of melanocytes into affected hair follicles and epidermis during embryonal development.76-78
In general, patients with piebaldism are healthy and do not have associated systemic abnormalities. However, the disorder occasionally has been associated with heterochromia irides, mental retardation, osteopathia striata, Woolf syndrome, and Hirschsprung disease.
Diagnosis
Cutaneous depigmentation is the only manifestation of piebaldism in 10% to 20% of cases. Amelanotic macules are usually present on the ventral surface of the thorax and abdomen and extend to the back but spare the midline. Characteristic extremity lesions extend from midarm to wrist and occur on the midleg [see Figure 5]. White patches of the mucous membranes have also been reported. Hyperpigmented macules may appear within the areas of depigmentation.
Light and electron microscopic studies of the white macules have typically revealed an absence of melanocytes. However, melanocytes have been demonstrated in the white forelock and amelanotic skin of three patients studied.
Differential Diagnosis
Piebaldism is sometimes confused with vitiligo, but in piebaldism, the leukodermic patches are both congenital and relatively static in shape and size.
Treatment
The lesions of piebaldism are usually stable throughout life, although some patients have reported spontaneous repigmenta-tion. In general, therapeutic approaches, including psoralen pho-tochemotherapy and grafting, are unsatisfactory. Autologous melanocyte grafting procedures may offer some benefit for localized or limited areas of involvement.
Idiopathic guttate hypomelanosis
Definition
Idiopathic guttate hypomelanosis (IGH) is a common asymptomatic disorder characterized by hypopigmentation and depig-mented polygonal macules ranging from approximately 2 to 8 mm in diameter.
Epidemiology
IGH appears to be a very common, benign dermatosis. It occurs in all races, with a frequency ranging from 46% to 70%, but is more prevalent in darker-skinned racial and ethnic groups. Macules may begin to appear during the third or fourth decade of life and gradually increase in number thereafter.
Etiology and Pathogenesis
The precise pathogenesis has not been established for IGH. Long-term sun exposure, trauma, genetic influences, and aging, with a gradual loss of melanocytes, have been implicated in the pathogenesis of this disorder.81
Diagnosis
The lesions of IGH are macules that are punctate to polygonal in shape, 2 to 8 mm in diameter, and hypopigmented to depig-mented. They are most commonly observed on the lower extremities. There is no atrophy or change in the overlying skin. Histologic evaluation of lesions reveals hyperkeratosis, epidermal atrophy, and decreased epidermal melanin. Melanocytes may be normal or decreased. Immunoperoxidase studies show a markedly reduced number of melanocytes. Melanocyte differentiation appears to be unaffected.82
Differential Diagnosis
IGH must be differentiated from other hypopigmentary disorders, such as vitiligo, scleroderma, leukodermic guttate para-psoriasis, tinea versicolor, hypopigmented sarcoidosis, pity-riasis alba, chemical depigmentation, and postinflammatory hypopigmentation.
Treatment
No definitive treatment is currently available. Patients often need reassurance regarding the banality of lesions. For patients concerned about the cosmetic appearance of lesions, clinicians have used camouflage, intralesional steroids, and topical pho-tochemotherapy. Localized superficial dermabrasion may offer some improvement.83