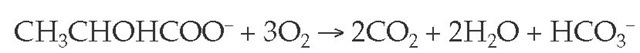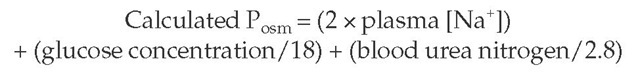Acid-Base Disorders
The blood pH is normally maintained at 7.38 to 7.42. Any deviation from this range indicates a change in the hydrogen ion concentration ([H+]) because blood pH is the negative logarithm of [H+], as expressed by the following equation:
The [H+] at a physiologic blood pH of 7.40 is 40 nEq/L [see Figure 1]. An increase in the [H+]—a fall in the blood pH—is termed acidemia. A decrease in the [H+]—a rise in the blood pH—is termed alkalemia. The disorders that cause these changes in the blood pH are acidosis and alkalosis, respectively. Because abnormalities of acid-base metabolism are often associated with potassium imbalance, clinical approaches to hypokalemia and hyperkalemia are also discussed in this topic.
Normal acid-base physiology
The normal adult diet generates an excess 70 to 100 mEq of acid that must be eliminated every day. Failure to do so results in a persistent fall in the blood pH resulting from a rise in the plasma H+ ion concentration. The balance of acid-base homeo-stasis is maintained in part by the relation between the arterial carbon dioxide tension (PaCO2) and plasma bicarbonate concentration ([HCO3-]), as noted in the following equation, a nonloga-rithmic expression of the Henderson-Hasselbach equation1:
A fall in plasma [HCO3-] caused by either gastrointestinal or renal bicarbonate losses also increases the [H+] and lowers blood pH.
Renal Reabsorption of Bicarbonate
The plasma [HCO3-] is normally maintained at approximately 25 mEq/L by the daily reabsorption of the filtered bicarbonate load (about 4,500 mEq) by the kidneys. If the filtered bicarbonate were not reabsorbed, the plasma [HCO3-] would fall, as would the blood pH. Thus, maintenance of a normal plasma [HCO3-] requires reabsorption of essentially all of the bicarbonate filtered across the glomerular capillaries each day.
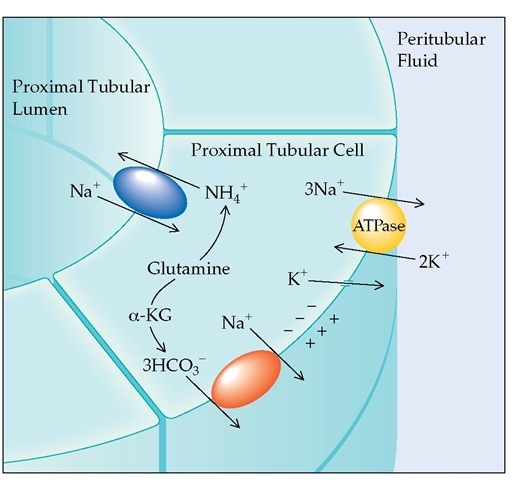
Most bicarbonate reabsorption (almost 90%) occurs in the proximal convoluted tubule [see Figure 2]; in contrast, the distal nephron reclaims very little bicarbonate. The difference is a result of a complex process, which is facilitated by the greater quantity of carbonic anhydrase in the lumen of the proximal tubule.
Renal Excretion of Acid
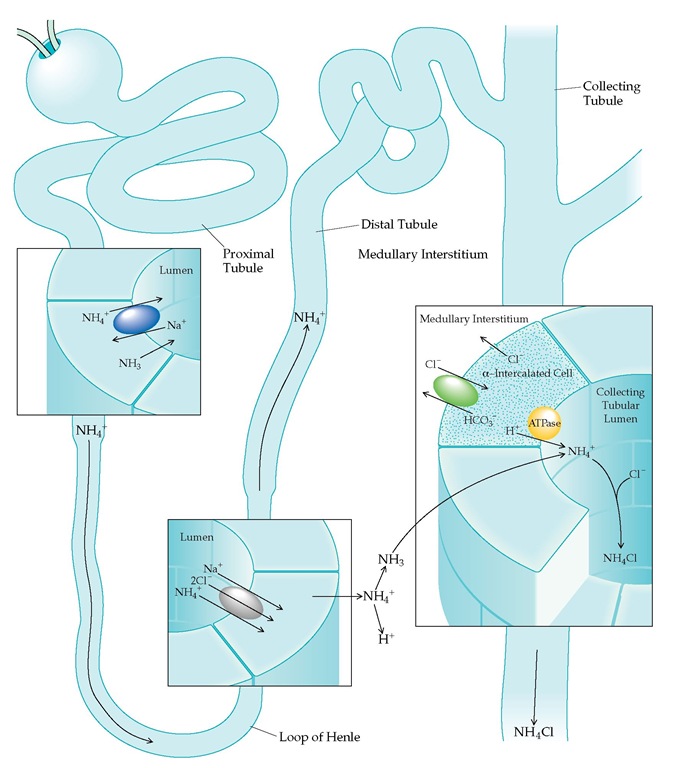
In addition to reabsorbing essentially all filtered bicarbonate, the kidneys excrete the daily dietary acid load, derived mainly from sulfur-containing amino acids. The hydrogen ions that are excreted in the final urine are secreted mainly in the collecting tubules [see Figure 3]. This secretory process is facilitated indirectly by aldosterone and directly by acid-sensing renal tubular cells.2
The daily acid load is excreted into the collecting tubules by the H+-ATPase pumps located in the luminal membrane of the intercalated cells. This secretory process is inhibited by a trivial quantity of free hydrogen ions that lower the urine pH below the critical level of 4.0 to 4.5. This limitation is normally overcome by the presence of urinary buffers that combine with free hydrogen ions, thus permitting continued secretion of acid. There are several urinary buffers, the most important of which is ammonia because it is the only buffer that can increase substantially in the presence of an acid load. Limitation of the capacity to generate adequate urinary ammonia, as occurs in renal insufficiency, usually leads to acidosis.
The major site of ammonia production in the kidney is the proximal tubule [see Figure 4]; ammonia moves from the proximal tubule to the collecting tubule, where it is eliminated [see Figure 5]. The quantity of ammonia produced is stimulated both by acidemia and by hypokalemia. Conversely, alkalemia and hyperkalemia limit renal tubular ammonia production and acid excretion.
Metabolic acidosis
Metabolic acidosis results whenever a primary decrease in the plasma [HCO3-] occurs. Such a decrease may be caused by several factors: exogenous acid administration, endogenous acid production, impaired renal hydrogen secretion, and bicarbonate losses from the kidney or in gastrointestinal secretions. Calculation of the plasma anion gap is particularly useful in identifying the specific cause of metabolic acidosis and in narrowing the differential diagnosis.
Figure 1 The relation between the plasma hydrogen ion concentration ([H+]) and the pH of the blood (pH = -log10 [H+]).
Figure 2 Proximal tubular bicarbonate reabsorption is activated by the Na+,K+-ATPase pump in the peritubular cell membrane. Exchanging peritubular K+ for intracellular Na+ keeps the intracellular [Na+] low, allowing Na+ to move down its concentration gradient from the tubular lumen through the Na+-H+ antiporter to the cell interior. HCO3- filtered across the glomerular capillaries combines with secreted H+ to form H2CO3. Rapid dissociation of H2CO3 to CO2 and H2O in the presence of luminal carbonic anhydrase permits movement into the cell, where redissociation occurs. Ultimately, the reabsorbed H+ is resecreted in exchange for Na+, and HCO3" moves down an electrical gradient from the cell interior to the peritubular space, where it is reabsorbed into the systemic circulation.
Anion Gap in Metabolic Acidosis
The anion gap (measured in mEq/L) refers to the difference between the plasma concentrations of the major measured cation (sodium) and the major measured anions (chloride and bicarbonate), as described by the following equation:
Anion gap = [Na+] – ([HCO3- ] + [Cl-])
The normal anion gap ranges from 3 to 13 mEq/L, with a mean of about 10 mEq/L. It is composed mainly of plasma proteins (primarily albumin) that carry a negative charge. In the setting of severe hypoalbuminemia, the baseline anion gap may be less than 3 mEq/L, because the anion gap falls by approximately 2.5 mEq/L for every 1 g/dl reduction in the serum albumin concentration.
The most important clinical use of the anion gap is to identify the etiology of metabolic acidosis.3 The disorders that cause metabolic acidosis fall into two categories: (1) those that cause a fall in the plasma [HCO3-] while concomitantly raising the anion gap and (2) those that cause a fall in the plasma [HCO3-] without affecting the anion gap. In the latter setting, the plasma [Cl-] increases, giving rise to a hyperchloremic metabolic acidosis.
Causes of Metabolic Acidosis with a High Anion Gap
Several disorders, as well as the ingestion of toxins, can cause metabolic acidosis with an increased anion gap [see Table 1].
Renal failure Advanced renal failure is the most common cause of metabolic acidosis with an increased anion gap in the outpatient setting. The retention of hydrogen ions leads to a fall in the plasma [HCO3-]. Because sulfate and phosphate (which are the accompanying anions) are excreted in the urine while chloride is retained, the anion gap remains normal during the early course of acidosis in renal failure. As renal failure progresses (creatinine > 3.0 mg/dl), these ingested anions and metabolic waste products can no longer be excreted normally. At this point, the anion gap increases. There is, however, no linear correlation between the degree of acidemia (or hypobicar-bonatemia) and the level of the anion gap. In uncomplicated renal failure, the plasma [HCO3-] rarely decreases to less than 12 mEq/L, and the anion gap characteristically remains less than 20 mEq/L.
Lactic acidosis Lactic acidosis is the most common cause of high-anion-gap metabolic acidosis observed in hospitalized patients. Lactic acid production usually increases as a result of hypotension or sepsis, both of which cause true or relative tissue is-chemia.
Figure 3 Secretion of H+ from the cortical collecting tubule is indirectly linked to Na+ reabsorption. Intracellular potassium is exchanged for sodium in the principal cells, whereas H+ is actively transported by an ATPase pump from the a-intercalated cells. Aldosterone stimulates H+ secretion by entering the principal cell, where it opens Na+ channels in the luminal membrane and increases Na+,K+-ATPase activity. The movement of cationic Na+ into the principal cells then creates a negative charge within the tubular lumen. K+ moves from the principal cells and H+ from the a-intercalated cells down this electrochemical gradient and into the lumen. (When K+ is depleted, principal cell K+ secretion is reduced, and K+ reabsorption via an ATPase pump in the a-intercalated cell is stimulated.) Aldosterone apparently also stimulates the H+-ATPase directly in the intercalated cell, further enhancing H+ secretion. HCO3 is returned to the blood across the peritubular membrane in exchange for Cl-, thus maintaining electroneutrality.
Figure 4 All of the ammonia used to buffer urinary H+ in the collecting tubule is synthesized in the proximal convoluted tubule, and glutamine is assumed to be the main source of this ammonia. As glutamine is metabolized, a-ketoglutarate (a-KG) is formed, which ultimately breaks down to bicarbonate that is then secreted into the peritubular fluid by an Na+-HCO3- cotransporter.
The oxidative pathways of pyruvate metabolism become markedly impaired in states of mitochondrial dysfunction, such as those induced by tissue hypoxemia. This setting enhances the conversion of pyruvate to lactate. The liver and, to a lesser degree, the kidneys are the major organs that remove lac-tate from the circulation. In both organs, lactate is converted back to pyruvate and then to carbon dioxide and water via the tricar-boxylic acid cycle. When normal tissue perfusion is restored, metabolism of lactate (CH3CHOHCOO-) in these organs rapidly regenerates the bicarbonate used initially to buffer the acid load, a process that is largely independent of renal acid excretion and that is summarized by the following equation:
The normal rate of lactate production can reach 320 mEq/hr (e.g., during exercise), a rate that is usually greater than the rate of lactate production in lactic acidosis. This finding indicates that for lactic acid to accumulate, lactate metabolism must also be impaired. In shock, for example, the marked reduction in hepatic perfusion slows lactate clearance.
Certain medications can also cause lactic acidosis—for example, metformin therapy in diabetics with renal failure and anti-retroviral therapy in people with AIDS.
The anion gap is almost always elevated above baseline in lactic acidosis. Because the renal excretory threshold for lactate is 6 to 8 mEq/L and the normal lactate level is less than 1 mEq/L, excess lactate accumulates in the blood, rather than being eliminated in the urine, thus contributing to the increased anion gap.
D-Lactic acidosis is an unusual form of lactic acidosis that is most often seen in patients who have undergone ileal bypass or small bowel resection.5 In each of these cases, short bowel syndrome can occur, resulting in increased bacterial metabolism of carbohydrate to D-lactic acid because of local overgrowth. In D-lactic acidosis, the anion gap rises initially but may fall over time because renal tubular reabsorption of D-lactate is inefficient in comparison with the reabsorption of L-lactate.
Ketoacidosis Acidosis caused by overproduction of the keto acids acetoacetic acid and |-hydroxybutyric acid occurs when insulin deficiency, fasting, or insulin resistance impairs glucose use. In these settings, ketone bodies are overproduced (a condition termed ketosis) and serve as an alternative source of energy for many cells. The initial ketone formed is acetoacetic acid, which may then be reduced to | -hydroxybutyric acid or nonen-zymatically decarboxylated to acetone. Although acetone is chemically neutral, the other ketones are organic acids, and their accumulation leads to metabolic acidosis. The following equation summarizes the reactions:
The anion gap characteristically increases in ketoacidosis. Acetoacetate and | -hydroxybutyrate are the major unmeasured anions that accumulate, although a concomitant lactic acidosis may be observed in some patients. The plasma [HCO3-] may be markedly depressed in diabetic ketoacidosis; in contrast, the acidemia is generally mild and the plasma bicarbonate rarely less than 18 mEq/L in starvation, or fasting, ketosis.
In patients with ketoacidosis, isotonic fluid replacement leads to ketonuria. At the initiation of fluid replacement, the anion gap is elevated, but it begins to fall as the rate of urinary excretion of acetoacetate and | -hydroxybutyrate exceeds the rate of production. Ultimately, normalization of the anion gap results at a time when the plasma [HCO3-] is still low.
Rhabdomyolysis Massive muscle breakdown is an important cause of metabolic acidosis.6 The retention of metabolic acids and such inorganic anions as phosphate appear to contribute to the increased anion gap. Metabolic acidosis is more likely to develop when concomitant renal failure is present [see 10:VI Acute Renal Failure].
Ingested agents and toxins Salicylates and the alcohols eth-ylene glycol (a component of antifreeze and solvents) and methanol, or wood alcohol (a component of shellac, varnish, de-icing solutions, and other commercial preparations), are the most frequent causes of metabolic acidosis among ingested agents and toxins. Ethyl alcohol ingestion is not associated with a high-anion-gap acidosis unless accompanied by either lactic acidosis or ketoacidosis.
The most common acid-base abnormality observed with sali-cylate intoxication in adults is respiratory alkalosis caused by direct stimulation of the medullary respiratory center [see Respiratory Acidosis and Alkalosis, below]. In adults, the presence of respiratory alkalosis appears to be a prerequisite for the development of metabolic acidosis, because an isolated metabolic acidosis is rare. Moderate to severe salicylate intoxication, on the other hand, leads to a mixed respiratory alkalosis and a high-anion-gap metabolic acidosis. Methanol and ethylene glycol can produce fatal intoxication, and both can cause a plasma osmolal gap, which refers to the difference between the plasma osmolali-ty (Posm) measured by the laboratory and that calculated by the following formula:
Figure 5 The ammonia used to buffer urinary hydrogen ions is synthesized in the proximal convoluted tubule. It then diffuses into the proximal tubular lumen or can become acidified within the cell, forming ammonium, which can enter the tubular lumen by substituting for hydrogen ions on the Na+-H+ antiporter. Ammonium flows through the thick ascending limb of the loop of Henle, where it is transported from the tubule into the medullary interstitium by replacing potassium on an Na+-K+-2Cl- transporter. In the interstitium, ammonium dissociates to ammonia, which diffuses down its concentration gradient into the lumen of the collecting tubule. Here, ammonia combines with secreted H+ to form ammonium; NH4+ is then excreted as NH4O to maintain electroneutrality. A bicarbonate molecule is regenerated for each H+ eliminated in the urine.
A high plasma osmolal gap (with the measured level at least 10 mOsm/kg higher than the calculated value) can be detected only when the Posm is measured by freezing-point depression. In contrast, the osmotic contribution of volatile alcohols is not included when a vapor-pressure osmometer, which assumes that only water is in the vapor phase, is used.7 Furthermore, the presence of an osmolal gap is not specific for these ingestions, and confirmation of the diagnosis requires plasma assays for the individual drugs [see Diagnosis, below].
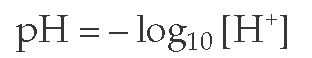
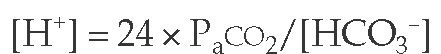
![The relation between the plasma hydrogen ion concentration ([H+]) and the pH of the blood (pH = -log10 [H+]). The relation between the plasma hydrogen ion concentration ([H+]) and the pH of the blood (pH = -log10 [H+]).](http://what-when-how.com/wp-content/uploads/2012/04/tmp8911_thumb.jpg)
![Proximal tubular bicarbonate reabsorption is activated by the Na+,K+-ATPase pump in the peritubular cell membrane. Exchanging peritubular K+ for intracellular Na+ keeps the intracellular [Na+] low, allowing Na+ to move down its concentration gradient from the tubular lumen through the Na+-H+ antiporter to the cell interior. HCO3- filtered across the glomerular capillaries combines with secreted H+ to form H2CO3. Rapid dissociation of H2CO3 to CO2 and H2O in the presence of luminal carbonic anhydrase permits movement into the cell, where redissociation occurs. Ultimately, the reabsorbed H+ is resecreted in exchange for Na+, and HCO3" moves down an electrical gradient from the cell interior to the peritubular space, where it is reabsorbed into the systemic circulation. Proximal tubular bicarbonate reabsorption is activated by the Na+,K+-ATPase pump in the peritubular cell membrane. Exchanging peritubular K+ for intracellular Na+ keeps the intracellular [Na+] low, allowing Na+ to move down its concentration gradient from the tubular lumen through the Na+-H+ antiporter to the cell interior. HCO3- filtered across the glomerular capillaries combines with secreted H+ to form H2CO3. Rapid dissociation of H2CO3 to CO2 and H2O in the presence of luminal carbonic anhydrase permits movement into the cell, where redissociation occurs. Ultimately, the reabsorbed H+ is resecreted in exchange for Na+, and HCO3" moves down an electrical gradient from the cell interior to the peritubular space, where it is reabsorbed into the systemic circulation.](http://what-when-how.com/wp-content/uploads/2012/04/tmp14212_thumb_thumb.jpg)