Disorders of Neuromuscular Transmission
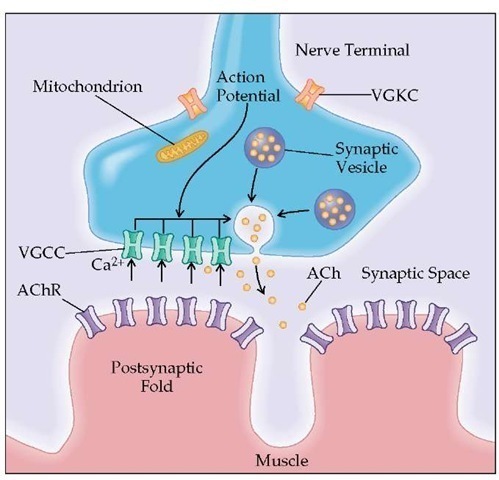
The main areas of the neuromuscular junction are (1) the presynaptic region, with active zones that contain parallel double rows of voltage-gated calcium channels (VGCCs) and synap-tic vesicles that contain acetylcholine (ACh), (2) the synaptic space, and (3) the postsynaptic region, consisting of the junction-al folds that contain the acetylcholine receptors (AChRs) [see Figure 3]. Functionally, neuromuscular transmission depends on the release of ACh from the motor nerve terminal, interaction of ACh with AChR, and the resulting depolarization of the muscle fiber. In a resting state, there is a random release of ACh from a single vesicle. ACh binds to AChR and opens its cation-selective ion channel, allowing Na+ to enter the end-plate region; this creates a depolarization potential called the miniature end-plate po-tential.89-91 ACh is then disassociated from AChR and rapidly hy-drolyzed by acetylcholinesterase (AChE). When AChE is inhibited, as occurs during treatment with such anticholinesterase drugs as pyridostigmine, more ACh molecules bind to AChR and open multiple ion channels.
When the action potential reaches the nerve terminal, the VGCC opens and Ca2+ ions flow into the nerve terminal. The entry of Ca2+ triggers the fusion and exocytosis of the synaptic vesicles and the release of ACh molecules (each vesicle contains 6,000 to 10,000 ACh molecules) that diffuse and spread over the junctional folds. The simultaneous release of ACh by the fusion of 50 to 300 vesicles results in the end-plate potential (EPP). If the EPP exceeds a certain threshold, a muscle action potential (MAP) triggers muscle contraction. Normally, the interactions between the released ACh and ACR are more than sufficient to produce an EPP capable of triggering an MAP. The difference between the actual EPP amplitude and the EPP amplitude required to trigger an MAP is called the safety margin.
There are three main disorders affecting neuromuscular transmission: myasthenia gravis (MG), the Lambert-Eaton my-asthenic syndrome (LEMS), and the various congenital myas-thenic syndromes.
Myasthenia gravis
Myasthenia gravis (MG) is a prototypical autoimmune disease; it fulfills all the immunologic criteria 89-91: the antigen is AChR; antibodies directed against AChR are detected and measured in the serum of affected patients; the disease can be transmitted to animals with the patient’s pathogenic IgG; serum immunoglobu-lin binds to AChR, causing a complement-fixing destruction of the receptors, which affects neuromuscular transmission; and removal of the antibodies results in clinical improvement.
In MG, antibodies against AChR reduce the number of available receptors. Consequently, the released ACh cannot bind to enough AChR; thus, the generated EPP is insufficient to trigger an MAP, and neuromuscular transmission fails. When neuro-muscular transmission failure occurs in many neuromuscular junctions, the muscle cannot be fully activated; this results in fatigue and muscle weakness. During repetitive nerve stimulation in healthy persons, the amount of ACh released is reduced after the first few impulses, because the nerve cannot sustain its original release rate; however, neuromuscular transmission does not fail, because the safety margin is sufficient. In patients with MG, the EPP is already small. During repetitive nerve stimulation, the EPP is depressed further because the decreasing amounts of ACh released per subsequent impulse are not sufficient to bind to the reduced number of AChR; this results in a further decrement of the MAP. Repetitive nerve stimulation studies are used diagnostically to demonstrate impaired neuromuscular transmission in MG patients. Because MG affects the nicotinic AChR at the neuromuscular junction, only motor systems are affected.
Epidemiology
The prevalence of MG is approximately four to six cases per 100,000 population. The annual incidence varies, but two peaks are recognized: one is associated with women in their second or third decade of life, and the other is mostly associated with men older than 60 years. The mean age at onset is 28 years in women and 42 years in men. There is recent documentation that MG is underdiagnosed in patients older than 70 years.92 Classic autoimmune MG can also develop during childhood and responds well to thymectomy or anticholinesterases. MG can be associated with thymoma in as many as 15% of adult patients, or it may develop after removal of a thymoma. In addition, as many as 5% of patients with MG have other systemic autoimmune conditions, as many as 15% of patients have various autoantibodies, as many as 20% of patients have thyroid disease, and as many as 5% of patients have a family history of another autoimmune disease. Pregnancy has a variable effect, but for most patients, weakness increases in the postpartum period. About 12% of children born to mothers with MG develop transient neonatal MG as a result of transplacental transfer of circulating anti-AChR antibodies from the myasthenic mother to the fetus. These infants develop weakness, feeding difficulties, respiratory problems, a weak cry, and facial weakness within the first few hours after birth. The condition lasts as long as 3 weeks, coinciding with the half-life of IgG.
Immunopathogenesis
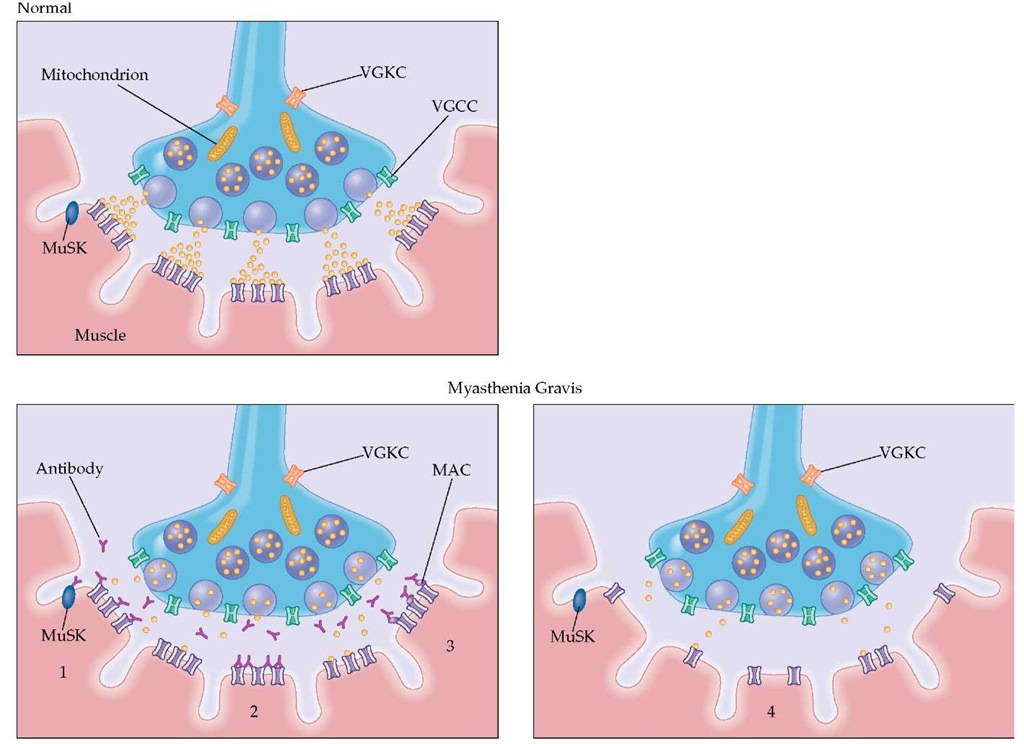
Autoantibodies MG is mediated by pathogenic autoanti-bodies that bind to AChR and cause a functional loss of the receptors and focal destruction of the postsynaptic junctional folds, interfering with the depolarization of the postsynaptic membrane [see Figure 4]. Between 10% and 15% of MG patients, especially those with mild, childhood, or localized (e.g., ocular) forms, do not have detectable antibodies. Most of these pa- tients, although seronegative, have an antibody-mediated disorder. Among the 15% seronegative patients, about 6% have antibodies against MuSK,93 a muscle-specific kinase needed for the development and clustering of the AChR at the neuromus-cular junction [see Figure 4]; the other 9% of seronegative patients contain poorly characterized IgG antibodies that re-versibly inhibit AChR function or IgM antibodies that indirectly inhibit AChR function.
Figure 3 Transmission from the nerve ending at the neuromuscular junction takes place at localized sites (active zones). The presynaptic region contains the voltage-gated calcium channels (VGCCs) and synaptic vesicles that hold 6,000 to 8,000 molecules of the neurotransmitter acetylcholine (ACh). When the action potential reaches the nerve terminal, the VGCCs open and Ca2+ flows into the nerve terminal. The entry of Ca2+ triggers the fusion and exocytosis of the synaptic vesicles. ACh is released into the synaptic space and binds to the acetylcholine receptor (AChR) located in the end-plate region of the muscle membrane (postsynaptic folds). Activation of AChR by ACh causes ions to flow through AChR across the membrane, initiating the electrical response of the muscle called the end-plate potential (EPP). The EPP spreads from the end-plate region to the surrounding muscle membrane and initiates the impulse response of the muscle that ultimately leads to muscle contraction.
Other antibodies associated with MG include antistriational cell antibodies. These are found in as many as 80% of patients with thymoma and MG, 25% of patients with thymoma without MG, 30% of adults with MG (55% when MG begins after age 60), 6% of patients with LEMS, 3% of patients with lung cancer, and frequently in patients with autoimmune liver disease.94 Anti-titin antibodies are also present in 55% of AChR-positive patients with late-onset MG, regardless of whether the patients have thy-moma. Antibodies to thyroid microsomes and thyroglobulin are frequently present in patients with ocular MG and in patients with LEMS without cancer.
Immunoregulation The AChR is a T cell-dependent antigen. In MG, the CD4+ cells are sensitized and respond to stimulation with AChR or to synthetic immunodominant peptides of AChR.89-92 Because lymphocytes from normal, healthy persons also respond to such peptides, albeit to a lesser degree, the AChR-specific T cells are part of the normal immune repertoire. This suggests that MG is a disease of abnormal im-munoregulation. Induction of MG by microbial antigens (e.g., antigens to Escherichia coli and Klebsiella) or herpes simplex viruses have been postulated because these bacteria and viruses share sequence homology with peptides of the alpha sub-unit of AChR.
Approximately 75% of patients with MG have thymic abnormalities, either hyperplasia with germinal center formation or thymoma (15% of all patients with MG). Removal of the thymus results in clinical improvement or remission. Additional evidence implicating a role for the thymus in MG is based on several findings: (1) the thymus of an affected person contains a greater population of B cells that could secrete AChR antibodies than the thymus of a normal, healthy person; (2) the thymus of an affected person possesses myoid cells that contain AChR proximal to the interdigitating dendritic cells; and (3) the number of T cells sensitized to AChR is greater in the thymus of an affected person than in the thymus of a healthy person.
In MG, breaking of self-tolerance may begin in the thymus. AChR on the myoid cells may thus be recognized by the thymic dendritic cells that present the antigen to CD4+ cells, which in turn stimulate the antibody-producing B cells to make anti-AChR antibodies. Subsequently, these antibodies recognize and cross-react with AChR in skeletal muscle.
MG is frequently associated with other autoimmune conditions, including collagen vascular diseases, polymyositis, pemphigus, autoimmune thyroid diseases, and graft versus host disease. The use of D-penicillamine has induced classic MG, which improves when the drug is discontinued.
Diagnosis
Clinical manifestations Patients with MG present with skeletal muscle weakness and fatigability. The weakness often affects elevation of the eyelids and movement of the extraocu-lar muscles, which causes a characteristic asymmetrical ptosis and diplopia. The neck extensors are often weak, and the head droops. Patients with MG may have weakness of facial and bulbar muscles that causes a facial snarl when a patient smiles; nasal or dysarthric and low-volume dysphonic speech; and dysphagia, which can result in regurgitation and choking. Proximal skeletal muscle weakness and fatigue produce difficulty in walking, climbing steps, combing hair, and carrying objects. Respiratory muscle weakness can be significant. Symptoms fluctuate and are often better in the morning. Fatigability is usually worse at the end of the day or after repeated or continuous activity. If a patient is asked to keep the arms abducted, a gradual decline in arm height is noted. If a patient is asked to talk continually, the voice may become husky, nasal, slurred, and finally inaudible. Sensation and cognition are normal. Tendon reflexes, especially from nonatrophic muscles, tend to be brisk or normally active. The toes are downgoing. The symptoms worsen during or before the menstrual period and during viral or bacterial infections.
Figure 4 Destruction of the AChR in myasthenia gravis by autoantibodies. The AChR autoantibodies are pathogenic and impair neuromuscular transmission (1) by blocking the available AChRs, thereby preventing the binding of ACh to the AChR (the functional significance of this effect is considered minimal); (2) by cross-linking the AChRs, thereby accelerating AChR internalization and proteolytic degradation by the mechanism of antigenic modulation; and by fixing complement, a process that leads to (3) deposition of the lytic membrane attack complex (MAC) on the AChRs, which results in (4) the progressive lysis of the membrane and shedding of the postsynaptic folds into the synaptic space. These processes lead to the simplification of the postsynaptic membrane and loss of AChRs. About 6% of patients without AChR antibodies have antibodies against MuSK, a muscle-specific kinase that is needed for the development and clustering of AChR. The pathogenic role of the anti-MuSK antibodies remains unclear.
Patients with MuSK antibodies tend to be women; disease onset in these patients occurs at 20 to 60 years of age. Frequently, patients present with prominent neck, shoulder, or respiratory muscle weakness and bulbar signs.95 Others present with predominantly oculobulbar signs.
Laboratory tests In addition to demonstration of fatigue and weakness on examination, the clinical diagnosis can be confirmed by an edrophonium or neostigmine test. These drugs inhibit AChE, which allows ACh to interact repeatedly with the remaining AChR, and as a result, muscle strength improves. Repetitive nerve stimulation studies demonstrate a rapid reduction (> 12%) in the amplitude of the MAP. Single-fiber EMG demonstrates increased jitter and blocking in more than 90% of patients. An MRI of the chest should be performed to search for thymic hyperplasia or thymoma. MG must be differentiated from diseases or exogenous agent-induced toxic disorders that have similar clinical pictures, such as botulism, organosphos-phate poisoning, D-penicillamine toxic reaction, mitochondrial myopathy, compressive lesion affecting cranial nerves, LEMS, and congenital myasthenic syndromes. The serologic diagnosis of MG is made by detecting AChR or MuSK antibodies with a radioimmunoprecipitation assay.90 The AChR antibodies are present in as many as 85% to 90% of patients with generalized MG and in as many as 70% of patients with ocular MG; they are also found in as many as 30% of patients with autoimmune liver disease, 10% of patients with pernicious anemia, and as many as 13% of patients with LEMS.96 Generally, AChR antibody titers do not correlate with clinical severity.
Treatment
The term gravis is now a misnomer because MG responds fairly well to the available therapies. Although the sequence in which the various therapeutic modalities are applied may differ among physicians, the initial choice in mild cases is generally an anticholinesterase drug given every 4 hours while the patient is awake. Thymectomy is done in most centers through the transsternal approach in patients beyond puberty and up to 55 years of age. The beneficial effect of thymectomy, however, has been questioned and has necessitated a large multicenter study.97 Thymoma should always be removed, followed by radiation therapy.
Prednisone is the first-line immunotherapeutic drug therapy. The preferred dosage is 60 to 80 mg as a single daily dose in the morning for an initial period of 3 to 4 weeks. Then, over an 8-week period, the dose on alternate days is gradually reduced by 10 mg a week (more often if side effects are severe) until the lowest dose that controls the disease is reached. Some physicians prefer to start with lower dosages of prednisone (i.e., 20 to 40 mg/day) and gradually increase the dosage to prevent an occasionally noted transient worsening. Plasmapheresis or intravenous immune globulin is generally reserved for crisis and severe cases and to strengthen a patient before thymectomy. Cyclosporine, mycophe-nolate, or azathioprine may be used as a second-line therapy to maintain remission after lowering the steroid dosage.89,90 Azathio-prine, for example, given orally in dosages of up to 3 mg/kg/day has been shown in a controlled study to be effective in maintaining remission of symptoms after more than 12 months of administration, permitting a reduction in steroid dose.
Patients with MuSK antibodies do not improve with thymec-tomy, and their response to cholinesterase inhibitors is variable. The response to immunotherapy is generally good but variable: some patients may develop permanent facial, pharyngeal, or tongue weakness and atrophy.
Lambert-eaton myasthenic syndrome
In LEMS, specific antibodies against VGCCs cause antigenic modulation and depletion of VGCCs [see Figure 4]. This restricts Ca2+ ingress into the motor nerve terminal and reduces ACh release during a nerve impulse. The amount of ACh released can generate only a small EPP that cannot trigger an MAP; this results in neuromuscular transmission failure and muscle weakness. An important observation in LEMS is its frequent association with small cell lung cancer (SCLC), which also expresses VGCC.
LEMS occurs more frequently in men than in women. Approximately 70% of men with LEMS have SCLC, as compared with 20% of women. LEMS may precede the detection of malignancy by up to 3 years. Malignancy is rare in persons younger than 40 years.
Patients present with proximal muscle weakness, increased fatigability, and transient ocular symptoms of ptosis or diplopia. Autonomic symptomatology, with dry mouth, dry eyes, sexual impotence, constipation, and abnormal pupillary reflexes, is frequent. Hyporeflexia is common. A typical sign is an increase in muscle strength and reflexes a few seconds after a sustained maximal effort. The diagnosis is confirmed by repetitive nerve stimulation studies that demonstrate a dramatic increase in the amplitude of the MAP upon tetanic stimulation. Single-fiber EMG shows increased jitter and blocking, which improve with faster stimulation rates. Using radiolabeled peptides from cone snail toxins, antibodies to P-type or Q-type calcium channels are detected in as many as 95% of LEMS patients.909199 100 Antibodies to N-type channels are seen in as many as 75% of LEMS patients, especially those who have lung cancer. A search for SCLC, especially in men older than 40 years, is an essential part of the patient workup. Antibodies to the P-type or Q-type VGCC are also found in patients with paraneoplastic cerebellar degeneration in both the serum and the CSF. These antibodies may interfere with calcium influx into cerebellar Purkinje cells and may be implicated in the patient’s ataxia.
The presynaptic region of the motor end plates in patients with LEMS exhibits marked depletion of the active zone particles that represent the VGCCs. The VGCCs in LEMS are no longer in parallel rows but are aggregated as a result of cross-linking by the specific IgG antibodies. The patient’s IgG [F(ab')2 fraction] injected into mice transmits the electrophysiologic and morphologic changes associated with the disease. Complement is not involved in the process. SCLC expresses VGCC of the N, L, or P type. It is believed that tumor cells trigger autoantibodies against surface determinants, which cross-react with similar epi-topes at the presynaptic regions of the neuromuscular junctions.
In LEMS associated with SCLC, the primary focus is to treat the tumor because the symptoms of LEMS improve as the cancer regresses. The first drug of choice in LEMS is 3,4-diaminopyri-dine. This drug prolongs the duration of the presynaptic action potential by blocking the outward K+ currents; this allows increased Ca2+ entry into the nerve terminal and further release of ACh. The drug alleviates fatigue and weakness in LEMS patients, and it is well tolerated in dosages of 40 to 60 mg daily. Sometimes, pyridostigmine is added. Prednisone helps substantially. Azathioprine, plasmapheresis, and intravenous immune globulin are additional therapeutic approaches that are given as steroid-sparing agents or to improve the strength of patients not responding well to 3,4-diaminopyridine and prednisone.
Neuromyotonia with k+ channel autoantibodies (isaac syndrome)
Isaac syndrome is a rare autoimmune disease presenting as isolated neuromyotonia [see Isolated Neuromyotonia (Isaac Disease), above] or in association with thymoma, MG, and CNS symptoms, such as limbic encephalitis, personality changes, hallucinations, and sleep disturbances.74 Patients with neuromyoto-nia have myokymia, myotonia, and, often, muscle hypertrophy. Myokymic discharges and prolonged motor unit discharges are seen electrophysiologically. Other patients have cramp-fascicu-lation syndrome and peripheral neuropathy.
An antibody against o-dendrotoxin-sensitive K+ channels is responsible for the disease.73,74 Affected patients have antibodies against voltage-gated potassium channels (VGKCs). The MAP is generated by the opening of the VGCCs; inactivation of the VGKCs, followed by the opening of the potassium channels, leads to repolarization of the nerve terminal. In patients with antibodies to VGKC, the depolarization is prolonged, prompting the calcium channel to remain open longer, so that more calcium enters the nerve terminal and more quanta are released.
This autoimmune disorder responds to plasma exchange, intravenous immune globulin, or immunosuppressants.
Congenital myasthenic syndromes
The congenital myasthenic syndromes are a heterogeneous group of syndromes that cause a failure of neuromuscular transmission that is not autoimmune, but, rather, is the result of genetic defects in various molecules, enzymes, or channels at the neuromuscular junction.
A congenital myasthenic syndrome is suspected when an infant, a child, or, occasionally, a young adult presents with fluctuating ptosis, fatigability, increased weakness on sustained exertion (common to all the neuromuscular transmission defects), mild or delayed motor milestones, absence of autoimmune MG, and a positive family history.101