An adverse drug reaction (ADR) is defined as any noxious, unintended, and undesired effect of a drug that occurs at doses used in humans for prophylaxis, diagnosis, or therapy.1 An ADR may range from a cutaneous eruption to severe syndromes (e.g., drug hypersensitivity syndrome, Stevens-Johnson syndrome [SJS], toxic epidermal necrolysis [TEN], and serum sickness-like reaction). Over the past 20 years, a dramatic shift has occurred in our understanding of drug-induced cutaneous eruptions. It is now believed that many severe cutaneous adverse drug reactions are caused by the formation of reactive ox-idative metabolites and perhaps the formation of antibodies to drug-protein complexes and skin proteins, cytochrome P-450 enzymes, or both. The predisposition to drug-induced eruptions may be genetic, and family counseling and in vitro testing are being used in certain centers to manage patients and their families. This topic reviews the pathophysiology and clinical manifestations that are important for correct diagnosis and treatment of cutaneous ADRs.
Epidemiology
Epidemiologic studies have shown that ADRs occur in 6.7% of all hospitalized patients,2 and 3% to 6% of all hospital admissions are the result of ADRs.3 In the Boston Collaborative Drug Surveillance Program,4 the prevalence of cutaneous ADRs in hospitalized patients was 2.2%. Antibiotics were responsible for 75% of detected reactions. In the Harvard Medical Practice Study, approximately 14% of ADRs in hospital patients were cutaneous or allergic in nature.5 The cost of drug-related morbidity and mortality has been estimated at $30 billion a year,6 and ADRs are thought to be between the fourth and sixth leading cause of death in the United States.2,6
Etiology
Cutaneous reactions to drugs often occur in complicated clinical scenarios that may include exposure to multiple agents. New drugs started within the preceding 6 weeks are potential causative agents, as are drugs that have been used intermittently, including over-the-counter preparations and herbal and naturopathic remedies.
Diagnosis
Clinical manifestations
The morphology of cutaneous eruptions may be exanthe-matous, urticarial, blistering, or pustular. The extent of the reaction is variable. For example, once the morphology of the reaction has been documented, a specific diagnosis (e.g., fixed drug eruption or acute generalized exanthematous pustulosis) can be made. The reaction may also present as a syndrome (e.g., serum sickness-like reaction or hypersensitivity syndrome reaction). Fever is associated with the more serious cutaneous ADRs.
Differential diagnosis
Differential diagnoses can include viral exanthems (e.g., infectious mononucleosis and parvovirus B19 infection), bacterial infections, Kawasaki syndrome, collagen vascular disease, and neoplasia.7
Laboratory tests
Penicillin skin testing with major and minor determinants is useful for confirmation of an IgE-mediated immediate hyper-sensitivity reaction to penicillin.8 Skin tests are performed 6 weeks to 6 months after complete healing of the cutaneous drug reaction.9 Oral rechallenges may be useful in the diagnosis of ADRs; however, they should not be used if a serious reaction, such as SJS or TEN, previously occurred. Patch testing may be helpful in the diagnosis of fixed drug eruptions or contact dermatitis.10
Exanthematous Eruptions
Simple eruptions
Exanthematous eruptions, also known as morbilliform, mac-ulopapular, or scarlatiniform eruptions, are the most common cutaneous ADRs.4 Simple exanthems are erythematous changes in the skin without blistering or pustulation.
Many drugs can cause exanthematous eruptions, including the penicillins, sulfonamides, barbiturates, antiepileptic medications, nonnucleoside reverse transcriptase inhibitors (e.g., nevirapine), and antimalarials.4,11 Exanthematous eruptions occur in 3% to 7% of patients receiving such aminopenicillins as ampicillin and amoxicillin. However, these eruptions may occur in 60% to 100% of patients taking ampicillin or amoxicillin who are receiving concurrent allopurinol therapy or who have concomitant lymphocytic leukemia, infectious mononucleosis, cytomegalovirus infection, or hyperuricemia.
Studies suggest that some exanthematous eruptions represent cell-mediated hypersensitivity.12,13 The etiology of the ampicillin rash concurrent with a viral infection is unknown, but the rash does not appear to be IgE mediated, and patients can tolerate all P-lactam antibiotics, including ampicillin, once the infectious process has resolved. A similar reaction was seen in 50% of HIV-infected patients exposed to sulfonamide antibiotics.14 Recent studies have shown that drug-specific T cells play a major role in exanthematous, bullous, and pustular drug reactions.15
Simple exanthems are symmetrical and often become generalized. Pruritus is the most frequently associated symptom. Fever is not associated with simple exanthematous eruptions. These eruptions usually occur within 1 week after the beginning of therapy and generally resolve within 7 to 14 days.16 The exanthem’s turning from bright red to brownish red marks resolution. Resolution may be followed by scaling or desquama-tion.17 Some patients with ampicillin- or amoxicillin-induced exanthematous eruptions may have a positive result on a patch test or on a delayed intradermal test.13,14 In general, however,skin testing is not considered helpful in the diagnosis of an ex-anthematous eruption.
The differential diagnosis of drug-induced exanthematous eruption includes viral exanthem (patients should be tested for mononucleosis), collagen vascular disease, bacterial infection, and rickettsial infection. Hypersensitivity syndrome should be considered in the differential diagnosis.
The treatment of simple exanthematous eruptions is generally supportive. For example, oral antihistamines used in conjunction with soothing baths may help relieve pruritus. Topical cortico-steroids are indicated when antihistamines do not provide relief. Systemic corticosteroids are used only in severe cases. Discontinuance of the offending agent is recommended in most cases.
Complex eruptions
Hypersensitivity Syndrome Reaction
Hypersensitivity syndrome reaction is a complex drug reaction that affects various organ systems. A triad of fever, skin eruption, and internal organ involvement signals this potentially life-threatening syndrome. It occurs in approximately one in 3,000 exposures to such agents as aromatic anticonvulsants (e.g., pheny-toin, phenobarbital, and carbamazepine), lamotrigine, sulfon-amide antibiotics, dapsone, minocycline, and allopurinol.18
It has been suggested that the metabolism of aromatic anti-convulsants by cytochrome P-450 plays a pivotal role in the development of the hypersensitivity syndrome reaction with these drugs.19 In most people, the chemically reactive metabolites that are produced are detoxified by epoxide hydroxylases. If detoxification is defective, however, one of the metabolites may act as a hapten and initiate an immune response, stimulate apoptosis, or cause cell necrosis directly.
In one study, 75% of patients with hypersensitivity syndrome reactions to one aromatic anticonvulsant showed in vitro cross-reactivity to the other two aromatic anticonvulsants.19 In addition, in vitro testing has shown that there is a familial occurrence of hyper-sensitivity to anticonvulsants.19 Although lamotrigine is not an aromatic anticonvulsant, it too can cause a hypersensitivity syndrome reaction.20,21 There is no evidence that lamotrigine cross-reacts with the aromatic anticonvulsants. Lamotrigine and other an-ticonvulsants are also associated with more severe reactions (e.g., SJS and TEN) [see Complex Eruptions, below].
Sulfonamide antibiotics can cause hypersensitivity syndrome reactions in susceptible persons. The primary metabolic pathway for sulfonamides involves acetylation of the drug to a nontoxic metabolite and renal excretion. An alternative metabolic pathway, quantitatively more important in patients who are slow acetylators, engages the cytochrome P-450 mixed-function oxidase system. These enzymes transform the parent compound to reactive metabolites—namely, hydroxylamines and nitroso compounds, which produce cytotoxicity independently of preformed drug-specific antibody. In most people, detoxification of the metabolite occurs. However, hypersensi-tivity syndrome reactions may occur in patients who are unable to detoxify this metabolite (e.g., those who are glutathione deficient).22 Although the detoxification defect is present in 2% of the population, only one in 10,000 people will manifest a hy-persensitivity syndrome reaction in response to sulfonamide antibiotics. Siblings and other first-degree relatives of patients with the detoxification defect are at increased risk (perhaps one in four) for having a similar defect.
Other aromatic amines, such as procainamide, dapsone, and acebutolol, are also metabolized to chemically reactive compounds. We recommend that patients who develop symptoms compatible with a sulfonamide hypersensitivity syndrome reaction avoid these aromatic amines, because the potential exists for cross-reactivity. However, cross-reactivity should not occur between sulfonamides and drugs that are not aromatic amines (e.g., sulfonylureas, thiazide diuretics, furosemide, and acetazolamide).
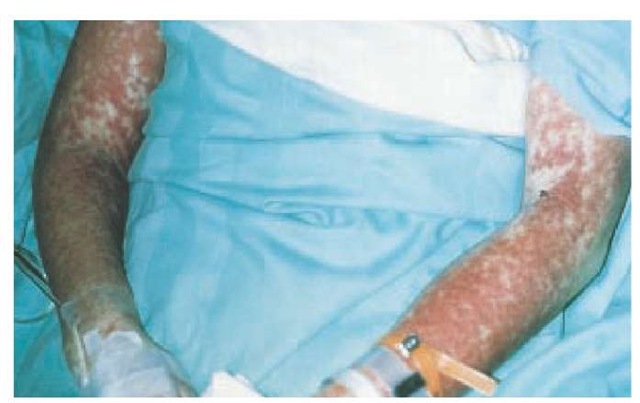
Hypersensitivity syndrome reaction occurs most frequently on first exposure to the drug, with initial symptoms starting 1 to 6 weeks after exposure [see Table 1]. Fever and malaise, which can be accompanied by pharyngitis and cervical lym-phadenopathy, are the presenting symptoms in most patients. This is often followed by edema and swelling of the face, especially upon rising in the morning. Atypical lymphocytosis, with subsequent eosinophilia, may occur during the initial phases of the reaction in some patients. A cutaneous eruption, which occurs in approximately 85% of patients, can range from an exanthematous eruption [see Figure 1] to the more serious SJS or TEN. The liver is often involved, resulting in hepatitis, although other internal organs may be affected, such as the kidney (e.g., interstitial nephritis and vasculitis), the central nervous system (e.g., encephalitis and aseptic meningitis), and the lungs (e.g., interstitial pneumonitis, respiratory distress syndrome, and vasculitis). A subgroup of patients may become hypothyroid as part of an autoimmune thyroiditis within 2 months after the initiation of symptoms.23
After hypersensitivity syndrome reaction has been recognized from the symptom complex of fever, rash, and lym-phadenopathy, some laboratory tests can be used to evaluate internal organ involvement, which may be asymptomatic. A complete blood count, urinalysis, and measurements of liver transaminase and serum creatinine levels should be per-formed.
Table 1 Clinical Features of Hypersensitivity Syndrome Reaction and Serum Sickness-like Reaction
|
Rash |
Fever |
Internal Organ Involvement |
Arthralgia |
Lymphadenopathy |
|
|
Hypersensitivity syndrome reaction |
Exanthem Exfoliative dermatitis Pustular eruptions Erythema multiforme Stevens-Johnson syndrome Toxic epidermal necrolysis |
Present |
Present |
Absent |
Present |
|
Serum sickness-like reaction |
Urticaria Exanthem |
Present |
Absent |
Present |
Present |
Figure 1 This 35-year-old woman developed hypersensitivity syndrome reaction, characterized by fever, rash, and hepatitis, 14 days after starting trimethoprim-sulfamethoxazole therapy. The rash is an extensive, symmetrical, red edematous eruption.
In addition, the clinician should be guided by symptoms that may suggest specific internal organ involvement (e.g., respiratory symptoms). Thyroid function should be evaluated on presentation of hypersensitivity syndrome reaction and then 2 to 3 months after presentation. A skin biopsy may help confirm the diagnosis when the patient has a blistering or a pustular eruption. Unfortunately, diagnostic or confirmatory tests are not readily available. An in vitro test employing a mouse hepatic microsomal system is used for research purposes to characterize patients who develop hypersensitivity syndrome reaction.19,24 Because of the severity of the reaction, oral rechallenges are not recommended.
Although the role of corticosteroids is controversial, most clinicians choose to start prednisone at a dosage of 1 to 2 mg/kg/day when symptoms are severe. Antihistamines, topical corticosteroids, or both can be used to alleviate symptoms. Because the risk of hypersensitivity syndrome reaction in first-degree relatives of patients who have had reactions is substantially higher than in the general population, counseling of family members regarding their risk of hypersensitivity syndrome reaction is advised.
Urticarial Eruptions
Simple eruptions
Urticaria and Angioedema
Urticaria is characterized by pruritic red wheals of varying sizes that can occur with any medication. When deep dermal and subcutaneous tissues are also swollen, the reaction is known as angioedema.
Urticaria and angioedema usually result from a type I immediate hypersensitivity reaction. This mechanism is typified by immediate reactions to penicillin and other antibiotics. Binding of the drug or its metabolite to IgE bound to the surfaces of cutaneous mast cells leads to activation, degranulation, and release of vasoactive mediators such as histamine, leukotrienes, and prostaglandins.25
Urticarial reactions may also result from nonimmunologic activation of inflammatory mediators. Drugs such as acetylsali-cylic acid and nonsteroidal anti-inflammatory drugs (NSAIDs),26 radiocontrast media, and narcotic analgesics27 may directly cause release of histamine from mast cells, independently of IgE. An-giotensin-converting enzyme (ACE) inhibitors are frequent causes of angioedema.28 The mechanism of this reaction is unclear but may relate to accumulation of bradykinin or activation of the complement system.
Although medications tend to cause urticaria, angioedema, or both, other causal agents are food [see 6:XVI Food Allergies], physical factors (e.g., dermatographism and cholinergic urticaria) [see 6:XIII Urticaria, Angioedema, and Anaphylaxis], and id-iopathic factors. Certain foods containing proteins that can cross-react with latex proteins, such as bananas, kiwifruit, avocados, and chestnuts, can cause oral itching and swelling, hives, or wheezing after ingestion. People at greatest risk for latex allergy include children with spina bifida and health care work-ers.29,30 Latex allergy can present as contact urticaria at sites of latex exposure, such as lip swelling in a person who has blown up a balloon or sucked on a pacifier. Contact with aerosolized powder from latex gloves to which the latex protein has adhered may cause mucosal symptoms, such as itchy, swollen eyes; runny nose; sneezing; or wheezing. Anaphylaxis may also occur.31
Signs and symptoms of IgE-mediated allergic reactions are typically pruritus, urticaria, cutaneous flushing, angioedema, nausea, vomiting, diarrhea, abdominal pain, nasal congestion, rhinorrhea, laryngeal edema, and bronchospasm or hypotension or both. Fever is not associated with urticaria or angioede-ma reactions. In general, individual lesions of urticaria last for less than 24 hours, although new lesions can continually develop. With ACE-inhibitor therapy, the onset of the adverse reaction is usually within hours but can occur as late as 1 week to several months into therapy.32 With treatment, the resulting an-gioedema usually resolves within 48 hours.
Skin testing may be helpful in cases of IgE-mediated urticaria. For example, penicillin skin testing with the major and minor determinants identifies approximately 99% of patients who have had an IgE-mediated reaction to penicillin.10 A latex skin test is a sensitive indicator of IgE sensitization.31 For large-molecular-weight agents, such as insulin,33 protamine,34 and egg-containing vaccines, positive immediate skin-test reactions identify patients at risk for IgE-mediated reactions.
Withdrawal of the causative agent is recommended. When angioedema or anaphylaxis occurs, immediate therapy with epinephrine and systemic steroids may be needed. Symptomatic relief can generally be achieved with antihistamines (H1 receptor blockers).
Differential Diagnosis
Allergic urticaria must be differentiated from urticaria caused by physical factors. Cold urticaria, for example, is precipitated by exposure to cold, occurring within minutes after immersion of hands or body in cold water or after exposure to cold air. In severe cases, systemic symptoms, including wheezing and syncope, can occur. A rare familial form of cold urticaria that is autosomal dominant has been linked to chromosome 1q44.35
Cold urticaria can be differentiated from other forms of urticaria by eliciting an urticarial reaction with an ice cube applied to the skin for 5 to 10 minutes. Other physical urticarias also have distinguishing causes or features. Solar urticaria occurs within minutes of exposure to sunlight and can be produced by exposing limited areas of skin to sunlight or to appropriate wavelengths of ultraviolet light in a phototherapy response to physical pressure. Cholinergic urticaria, which is characterized by small urticarial papules, can be induced by exposure to heat or by exercise.
Histologically, all the urticarias are characterized by an increase in mast cells in the dermis. Edema, vascular changes, and mononuclear infiltrates are more striking in the dermis of patients with cold urticaria. Mononuclear infiltrates are also more prominent in the deep dermis of patients with delayed pressure urticaria.36
As with drug-induced urticaria, first-line therapy of most urticarias consists of oral antihistamines and avoidance of precipitating factors. Psoralen plus ultraviolet A (PUVA) has been used successfully to treat patients with solar urticaria. Mon-telukast has been used successfully to treat delayed pressure urticaria,37 and cyclosporine is promising for cases of severe refractory chronic urticaria.38
Complex eruptions
Serum Sickness-like Reactions
Serum sickness-like reactions are defined by fever, rash (usually urticarial), and arthralgias occurring 1 to 3 weeks after drug initiation. Other symptoms, such as lymphadenopathy and eosinophilia, may also be present. In contrast to true serum sickness, serum sickness-like reactions are without immune complexes, hypocomplementemia, vasculitis, and renal lesions [see Table 1].
Epidemiologic studies in children suggest that the risk of serum sickness-like reactions is greater with cefaclor than with other antibiotics, including other cephalosporins.39,40 The overall incidence of cefaclor serum sickness-like reactions has been estimated to be 0.024% to 0.2% per course of cefaclor prescribed.
Although the pathogenesis is unknown, it has been postulated that in genetically susceptible hosts, metabolism of cefaclor produces a reactive metabolite that may bind to tissue proteins and elicit an inflammatory response that manifests as a serum sickness-like reaction.
Other drugs that have been implicated in serum sickness-like reactions are cefprozil,42 bupropion,43 and minocycline.18,44 The incidence of serum sickness-like reactions caused by these drugs is unknown.
Discontinuance of the culprit drug and symptomatic treatment with antihistamines and topical corticosteroids are recommended for patients with serum sickness-like reactions. A short course of oral corticosteroids may be required for patients with more severe symptoms. The drug that caused the serum sickness-like reaction should be avoided. For cefaclor and cef-prozil, the risk of cross-reaction with P-lactam antibiotics is small, and the administration of another cephalosporin is usually well tolerated.45 However, some clinicians recommend that patients who experience serum sickness-like reactions from ce-faclor avoid all P-lactam drugs.