Bleeding or bruising that is spontaneous or excessive after tissue injury may be caused by coagulation defects, fibrinolysis, abnormal platelet number or function, abnormal vascular integrity, or a combination of these abnormalities. This topic addresses hemorrhagic disorders associated with abnormalities in coagulation. Hemorrhagic disorders associated with quantitative or qualitative platelet abnormalities and disorders associated with blood vessels are discussed elsewhere [see 5:X1II Platelet and Vascular Disorders].
Disorders of coagulation may be inherited or acquired. Congenital coagulation disorders are rare and are most frequently caused by defects in single coagulation proteins, with the two X-linked disorders—factor VIII and factor IX deficiencies—accounting for the majority of defects. Acquired coagulation disorders are more common than the inherited disorders and are more complex in their pathogenesis. The most common acquired hemorrhagic disorders are vitamin K deficiency, drug-induced hemorrhage, and disseminated intravascular coagulation (DIC).
Hereditary Coagulation Disorders
The coagulation disorders appear clinically as either spontaneous hemorrhage or excessive hemorrhage after trauma or surgery. The patient history usually indicates whether the disorder is congenital or acquired. The hereditary disorders are characterized by their appearance early in life and by the presence of a single abnormality that can account for the entire clinical picture.
Von Willebrand Disease
Pathophysiology von Willebrand disease (vWD), the most common hereditary bleeding disorder, is caused by a deficient or defective plasma von Willebrand factor (vWF). The gene encoding vWF is on chromosome 12. vWF has specific domains for binding clotting factor VIII, heparin, collagen, platelet GPIb, and platelet GPIIb-IIIa. These domains relate directly to the following functions of vWF: (1) its action as a carrier molecule for factor VIII:C, in which it protects the clotting factor from proteolysis and substantially prolongs its plasma half-life; (2) its promotion of primary platelet adhesion at high wall shear rates by linking platelets via their GPIb-IX-V receptor to subendothelial tissues at the wound site; and (3) its support of platelet aggregation by linking platelets via their GPIIb-IIIa receptors.1 The vWF circulates as multimers that range in size from 0.5 million daltons (the dimer) to 20 million daltons. Even larger noncirculating multimers are present in endothelial cells, where they are stored in the Weibel-Palade bodies. The ultralarge vWF multimers are normally processed by the ADAMTS13 metalloprotease into smaller multimers as they are released from the endothelial cells. The vWF is released either into the circulation or abluminally, where it attaches to subendothelial collagen. Platelet a-granules also contain vWF, which is released when platelets are activated. The vWF multimers that are 12 million daltons or larger are the most effective in supporting platelet adhesion.
Laboratory Evaluation
The many variant forms of vWD differ in their clinical manifestations, laboratory abnormalities, and required therapies. Because vWF is a carrier protein for factor VIII, the activated partial thromboplastin time (aPTT) is prolonged when the vWF level is low. The platelet count is usually, but not invariably, normal. Bleeding time is generally prolonged but not sufficiently reliable to be used for diagnosis. An automated platelet function test utilizing a platelet function analyzer (PFA-100) has been shown to be a better screening test for vWD than the bleeding time.2,3 In this assay, citrated whole blood is aspirated through a capillary tube under high shear rates onto a membrane coated with collagen in which a central aperture is made. Platelets are activated by either adenosine diphosphate (ADP) or epinephrine. The closure time is a measure of platelet-vWF interaction.
The diagnosis of vWD requires the determination of factor VIII and vWF levels. There are two caveats: (1) laboratory testing is notoriously variable and (2) the patient’s blood group affects the vWF level—that is, patients with blood group O have lower vWF levels than those with blood group A, B, or AB, by as much as 30%.4 The vWF level is measured by either immunologic or functional methods. The former is reported as a percentage of normal vWF antigen. Because vWF circulates in physiologically important multimeric forms, it is sometimes helpful to determine the multimeric composition of the vWF in the patient’s plasma. This is especially useful in identifying type 2 vWD (see below). The functional level of vWF is tested by the ristocetin-in-duced platelet aggregation test. Ristocetin is added to a patient’s platelet-rich plasma, where it causes vWF to bind to platelets via the GPIb-IX-V receptor, leading to platelet activation and aggregation. In some laboratories, formalin-fixed platelets are used and, after the addition of ristocetin, agglutination of fixed platelets is measured.
Clinical Variants
The classification scheme for variants of vWD comprises three major groups: type 1 is a partial quantitative deficiency of vWF, type 2 is a qualitative abnormality of vWF, and type 3 is a severe and virtually total quantitative deficiency of vWF [see Table 1].5
Type 1 Type 1 vWD is the most common form of vWD, accounting for 75% of cases. It is generally an autosomal dominant trait that usually appears in the heterozygous form. In many cases, a mutation in the vWF protein occurs such that the mutant provWF monomers form dimers normally with the wild-type provWF monomers, but the resulting dimers are trapped in the endoplasmic reticulum and cannot be secreted.6 Patients with classic type 1 vWD have a lifelong history of mild to moderate bleeding, typically from mucosal surfaces. They may be unaware of a bleeding disorder until they undergo surgery or experience trauma, when bleeding may be severe. vWF antigen, factor VIII, and the ristocetin cofactor levels are all decreased.
Type 2 Type 2 vWD is characterized by qualitative abnormalities of vWF and a variable decrease in vWF antigen, factor VIII, and ristocetin cofactor. In type 2A, the largest multimers are absent; in type 2B, multimers bind excessively to platelets be cause of a gain-of-function mutation; in type 2M, the abnormal vWF does not bind to GPIb-IX-V; and in type 2N, the binding site of vWF for factor VIII is mutated. All type 2 vWD variants, except for the rare type 2M, are characterized by a loss of the high-molecular-weight vWF multimers in the VWF polymer analysis.
Table 1 Classification and Differentiation of von Willebrand Disease
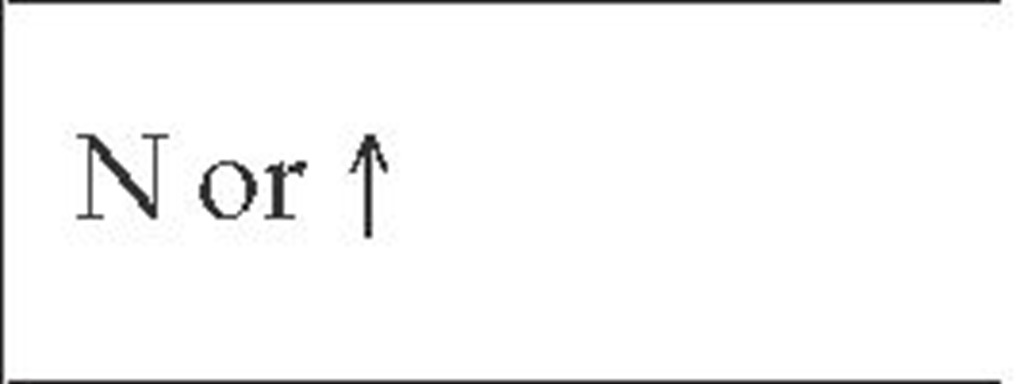
N—normal—decreased
Type 3 The rare homozygous or double heterozygous form (type 3 vWD) is characterized by severe hemorrhage, a long aPTT, and factor VIII levels of less than 5%.
Pseudo-von Willebrand disease A platelet form of vWD, which is termed pseudo-von Willebrand disease (pseudo-vWD), has been described in which an abnormal GPIb is present on platelets, causing excessive binding of normal plasma vWF to unstimulated platelets.
The mean level of vWF antigen is 100 IU/dl, but the population distribution of vWF levels is very broad, with the 95% values encompassing 50 IU/dl to 200 IU/dl. The reasons for this broad distribution in vWF levels are not completely understood, but it makes the commonly used threshold (vWF level at 2 SD [standard deviation] below the mean) inadequate for diagnosis of type 1 vWD. This problem with diagnosis is compounded by the fact that mild bleeding symptoms are extremely common in the general population. A recent survey estimates that 25% of men and 46% of women would give a positive history of bleeding symptoms, such as frequent epistaxis, easy bruising, and postpartum bleeding.7 This suggests that type 1 vWD may be overdiagnosed, and it has been proposed that a more stringent diagnostic criterion be used—namely, limiting the diagnosis of type 1 vWD to patients with a vWF antigen level of less than 20 IU/dl.6 Patients with modestly reduced vWF antigen levels (i.e., between 30 and 50 IU/dl) usually do not have identifiable vWF gene mutations and rarely cosegregate with a family history of bleeding. Patients with modestly reduced vWF levels (and no history of family bleeding) may have a modestly increased risk of bleeding.
Treatment
Mild or moderate types 1 and 2 1-Desamino-8-D-arginine vasopressin (DDAVP or desmopressin) is effective in the management of traumatic bleeding and before surgery in some patients with mild or moderate type 1 and type 2A vWD. The intravenous administration of DDAVP at a dosage of 0.3 mg/kg over a 15- to 30-minute period causes the release of large amounts of vWF from endothelial cell stores. The peak response usually occurs in 30 to 60 minutes and persists for up to 4 to 6 hours. Repeated DDAVP administrations over a 24-hour period are ineffective; tachyphylaxis follows depletion of the endothe-lial vWF stores. A DDAVP nasal spray (300 Mg) can be used in the ambulatory treatment of patients with vWD, both for the management of bleeding episodes and as preparation for minor surgery.8 The side effects of intravenous DDAVP are generally mild, including significant water retention and, rarely, thrombosis. Myocardial infarction has been reported. Because of the variability of response to DDAVP, a patient should be given a trial infusion of DDAVP before undergoing a planned procedure to determine whether the patient has an adequate response. Fibri-nolysis inhibitor E-aminocaproic acid (EACA), 3 g four times daily orally for 3 to 7 days, is also useful for dental procedures and minor bleeding events. Aspirin must be avoided.
Moderate and severe types 2 and 3 Patients with severe types 2A and 2B and with type 3 vWD generally require replacement therapy with Humate-P—a pasteurized intermediate-purity factor VIII concentrate that has a substantial amount of large vWF multimers—or with cryoprecipitate infusion containing vWF, factor VIII, and fibrinogen. Cryoprecipitate is generally not recommended. Transfusion of normal platelets can also be attempted on the grounds that platelet vWF can be hemostatically effective.
Treatment during pregnancy Treatment is generally not needed during pregnancy in women with vWD. The plasma vWF level rises during the second and third trimesters but falls rapidly after delivery. Late hemorrhage may occur 2 to 3 weeks post partum.10 DDAVP is not used before delivery because of the concern that it may initiate contractions. Patients with type 2B vWD may have worsening thrombocytopenia during pregnancy because of the increase of abnormal vWF in plasma.
Hemophilia A
Hemophilia A affects one in 10,000 males and is characterized by a deficient or defective clotting factor VIII. The factor VIII gene, which is located on chromosome X at Xq28, is among the largest known human genes, spanning 186 kb and containing 26 exons. It encodes a protein of about 300,000 daltons, which circulates in plasma at very low concentrations and is normally bound to and protected by vWF. The primary source of factor VIII production is likely the liver, because hemophilia A can be corrected by liver transplantation.
Because the gene for factor VIII coagulant activity is carried on the X chromosome, the disease is manifested in hemizygous males. All of the daughters of a hemophiliac male will be carriers, whereas half of the sons of a mother who carries the hemophilia trait will be hemophiliacs and half of her daughters will be carriers. Families appear to be affected to varying degrees, depending on the specific nature of the genetic defect.
The clinical severity of hemophilia A correlates well with the measured levels of factor VIII coagulant activity. In general, factor VIII levels below 1% are associated with severe hemorrhagic symptoms; levels between 1% and 5%, with moderate hemophilia; and levels between 5% and 25%, with mild hemophilia [see Table 2].
Approximately one third of hemophilia A cases represent new mutations and have a negative family history. More than 300 abnormal factor VIII genes have been found. The abnormalities, which include point mutations, gene insertions, and gene deletions, result in either deficient factor VIII production or the generation of a functionally defective factor VIII. An inversion within intron 22 of the factor VIII gene, which results in a truncated and unstable factor VIII protein, is found in approximately 45% of all severely affected hemophilia A patients (factor VIII levels below 1%).12
Diagnosis
Diagnosis is made on the basis of the clinical picture, family history (positive in two thirds of cases), and the factor VIII coag-ulant activity level. In most cases, the type of bleeding history and a classic family history rule out vWD (which, unlike hemophilia A, is autosomally transmitted). Accurate DNA analysis for the common intron 22 inversion is now available in DNA testing laboratories. This test provides molecular diagnosis in approximately 45% of patients with severe hemophilia. However, it should not be ordered in patients with mild or moderate hemophilia.
Table 2 Correlation of Factor VIII Coagulant Activity Level with Bleeding Patterns in Hemophilia
|
Plasma Factor VIII Level |
Bleeding Pattern |
|
<1% |
Severe, presentation in first year of life, bleeding with circumcision, spontaneous hemarthrosis and deep-tissue bleeding |
|
1%-5% |
Moderate, presentation in childhood, bleeding after trauma, spontaneous hemarthrosis rare |
|
5%-25% |
Mild; may be present in childhood; bleeding after trauma, surgery, or dental extraction |
|
25%-50% |
May be undetected, may present in adulthood with bleeding after major trauma or surgery |
Treatment
General principles The psychosocial aspects of hemophilia are complex. A child is often absent from school, is prone to crippling deformities, and runs a risk of drug addiction because of severe pain. Parents are understandably deeply concerned and sometimes troubled by guilt. Treatment should address these issues as well as the specific coagulation problem.
Factor VIII replacement Factor VIII concentrates are effective in controlling spontaneous and traumatic hemorrhage. Currently available factor VIII products derived from plasma have been purified to varying degrees (e.g., Humate-P [intermediate purity], Koate-HP [high purity], and Monoclate [ultrapurity]) and have undergone viral inactivation. There are two forms of full-length recombinant factor VIII (Recombinate and Kogenate), and they are safe and efficacious.13,14 A second-generation, B-do-main-deleted recombinant factor VIII (ReFacto) has also been developed and has been found to be effective and well tolerat-ed.15 The new recombinant factor VIII has the advantage of considerably higher specific activity, and the final formulation is stable without added human serum albumin, thus further reducing the potential risk of transmission of human infectious agents.
Dental prophylaxis is critically important to reduce the need for dental surgery. Aspirin must be avoided. Revaccination against hepatitis B virus also should be considered.
Genetic counseling should be part of the management program. Because of the difficult life severe hemophiliacs lead, a woman may opt to terminate pregnancy if she is certain of her carrier status or if she knows that her fetus is affected. There are several strategies for detecting carriers. In women who are carriers, factor VIII levels are typically about half of normal, whereas vWF levels are normal. The ratio of factor VIII to vWF for carriers is thus 0.516; however, the error rate for this test is 10% to 17%. A more accurate genetic diagnosis for carriers can be made by a linkage approach. This approach is based on restriction fragment length polymorphisms (RFLPs) within the factor VIII gene. Analysis of the affected male will establish the pattern for the X chromosome carrying the hemophilia allele, without knowledge of the precise mutation. There are a large number of intragenic polymorphisms that allow the two copies of factor VIII genes in a female potential carrier to be distinguished, identifying her carrier state with high accuracy.
These molecular probes for RFLPs are now being used to determine the status of the fetus. Tissue can be obtained by amnio-centesis or chorionic villus sampling.
Management of acute hemorrhage Deep tissue bleeding, hemarthrosis, and hematuria are the common forms of clinical bleeding in hemophilia A. Acute threats to life are posed by retroperitoneal hemorrhage; bleeding of the mouth, tongue, or neck that impairs the airway; and intracranial hemorrhage. Both ultrasonography and computed tomography can be used to identify retroperitoneal and intramuscular hematomas.
Principles of replacement therapy A plasma procoagulant level of 100% means that there is one unit of procoagulant per milliliter of plasma. Most persons have 40 ml of plasma per kilogram of body weight. Thus, from a determination of a patient’s plasma volume and procoagulant level, the required amount of factor VIII replacement can be calculated. For example, in the case of a 60 kg boy who has an uncomplicated hemarthrosis of the knee and a baseline factor VIII of less than 1%, raising the factor VIII level to about 25% (0.25 U/ml) for 2 to 3 days should suffice. This patient has a plasma volume of 60 kg x 40 ml/kg, or 2,400 ml; he will need 0.25 U/ml x 2,400 ml, or 600 U of factor VIII, as an initial bolus. Another method of estimation is based on the following effect: the infusion of 1 U of factor VIII per kilogram increases factor VIII levels by 2%. Thus, dividing the desired level of factor VIII increase by 2 will give the number of U/kg required. In the example cited, 25% of factor VIII will require 12.5 U/kg, or 750 U, of factor VIII replacement.
The biologic half-life of factor VIII is approximately 12 hours; the dose can be repeated every 12 to 24 hours as long as needed to control the hemorrhage. In patients with hemarthrosis, the factor VIII level should be maintained for 2 to 3 days.
Elective surgery and dental extraction Dental work should be performed by a dentist who is experienced in the treatment of hemophiliacs. Before dental extraction, factor VIII is administered to raise the level to approximately 50%. The fibrinolytic inhibitor EACA is started the night before surgery at a loading dose of 3 g orally and continued at 2 to 3 g three or four times daily for 7 to 10 days after the dental work has been completed. Usually, further administration of factor VIII is not required.
Before elective surgery, the factor VIII level should be raised to 50% to 100% (0.5 to 1.0 U/ml) and then maintained above 50% for the next 10 to 14 days. Maintaining a higher concentration of factor VIII does not reduce the frequency of hemorrhage.
DDAVP can be used to treat acute traumatic hemorrhage in patients with mild to moderate hemophilia and even to prepare such patients for minor surgery. DDAVP, which causes the release of vWF from endothelial cell stores, cannot be used repeatedly over many days, because such stores become depleted. DDAVP is infused at a dosage of 0.3 Mg/kg in 50 ml of saline over 15 to 30 minutes and produces a prompt increase in factor VIII. The biologic half-life of the released factor VIII is 11 to 12 hours.
Management of an inhibitor Inhibitors tend to occur in more severely affected patients, who tend to receive the greatest number of factor VIII concentrates. In a recent single-center study of 431 patients over 3 decades, approximately 10% of patients with severe hemophilia A had an inhibitor (about a third were children younger than 10 years).18 Not all inhibitors produce clinical problems. Assays for factor VIII inhibitors should be performed at regular intervals in all patients who have severe hemophilia.
Hemorrhage in a patient with an inhibitor can be life threatening. In a patient who has an inhibitor titer of less than 5 Bethesda units and who is not a vigorous antibody responder, a large amount of factor VIII concentrate should be administered in an attempt to overwhelm the antibody. Alternative therapies are porcine factor VIII (Hyate:C), prothrombin complex concentrates (e.g., Konyne and Proplex) to circumvent the factor VIII deficiency,19,20 and activated prothrombin complex concentrates, such as Autoplex-T and FEIBA.
Recombinant activated factor VII (rFVIIa) has been found to be safe and efficacious in 70% to 85% of more than 1,500 bleeding episodes in hemophilia patients with inhibitors.21,22 Recombinant factor VIIa may compete against the normal plasma unacti-vated factor VII for tissue factor binding and thus enhance thrombin generation at the bleeding site.23 In addition, high-dose rFVIIa may bind to activated platelets and activate factors IX and X on the platelet surface in the absence of tissue factor.
High-dose intravenous IgG has been used to treat nonhemo-philiacs with acquired factor VIII inhibitors, but it is usually not efficacious in hemophiliacs with inhibitors (alloantibodies).
Other Hereditary Hemorrhagic Disorders
Factor IX Deficiency (Hemophilia B)
Factor IX deficiency (hemophilia B, or Christmas disease) is an X-linked disorder that is clinically indistinguishable from hemophilia A. The factor IX gene is on the X chromosome and produces a clotting factor that, like other vitamin K-dependent factors, has a region rich in y-carboxylated glutamic acids. Calcium ion bridges link this region to the activated platelet cell surface, where factor IXa interacts with factor VIIIa to form a membrane-associated complex that efficiently converts factor X to factor Xa (intrinsic tenase) [see 5:XII Hemostasis and Its Regulation]. A large number of insertions, rearrangements, and deletions have been detected in the factor IX gene, and the hemophilia B syndrome is very heterogeneous.
Diagnosis Diagnosis of hemophilia B requires a factor IX assay. The management principles are the same as those for hemophilia A. A plasma-derived pasteurized factor IX concentrate preparation (Mononine) displays excellent specific activity and a desirable biologic half-life of 18 to 34 hours. Recombinant factor IX is also commercially available.
Treatment The level of factor IX that is needed to control he-mostasis in patients with hemophilia B is somewhat lower than the level of factor VIII required for the treatment of hemophilia A—about 15% to 20% for the former and 30% to 50% for the latter. Factor IX is a smaller molecule than factor VIII and is distributed in the albumin space. In making replacement calculations, it is assumed that administration of 1 U/kg of factor IX will increase the plasma level by 1%. Factor IX has a biphasic half-life, and plasma levels of this factor can be maintained by infusing the concentrate every 24 hours. Molecular biology techniques can now detect the factor IX deficiency carrier state and permit accurate genetic counseling. Sustained correction of a bleeding disorder in hemophilia B mice has been demonstrated by the gene therapy approach,26 and clinical trials of factor IX in hemophilia B patients have been initiated.