Cirrhosis is the most advanced stage of most types of chronic liver disease. It is defined as a diffuse disorganization of normal hepatic structure by extensive fibrosis associated with regenerative nodules. Fibrosis is potentially reversible if the causative agent is removed. However, advanced cirrhosis comprises major alterations in the hepatic vascular bed and is usually irreversible.1 Clinically, cirrhosis is associated with high morbidity and mortality. It leads to a wide spectrum of characteristic clinical manifestations, mainly from hepatic insufficiency and portal hypertension.2 Major complications include as-cites, renal failure, gastrointestinal bleeding, encephalopathy, bacterial infections, and coagulopathy. Cirrhosis is also a risk factor for developing hepatocellular carcinoma (HCC). Decom-pensated cirrhosis carries a poor prognosis, in both the short and the long term, and orthotopic liver transplantation (OLT) is often indicated.
Epidemiology
Cirrhosis is the ninth leading cause of death in the United States.3 Chronic liver disease and cirrhosis cause 4% to 5% of deaths in persons 45 to 54 years of age and result in about 30,000 deaths each year. The incidence of newly diagnosed cases of chronic liver disease in the United States is 72.3 per 100,000 population. The prevalence of chronic liver disease and cirrhosis is 5.5 million cases. Over 60% of patients are male. Cirrhosis is more common in Hispanic whites and Native Americans; it is the sixth leading cause of death in those two populations. The economic impact of cirrhosis is considerable, with $1.5 billion in direct costs and $234 million in indirect costs in 2000.4 In 2002, there were 421,000 hospitalizations for chronic liver disease and cirrhosis.
Etiology and Genetic Factors
In the United States, the main causes of cirrhosis are hepatitis C virus (HCV) infection and alcoholic liver disease, which account for two thirds of all cirrhosis cases. Other major causes are hepatitis B virus (HBV) infection, autoimmune hepatitis, chronic cholestasis (primary biliary cirrhosis [PBC] and primary scleros-ing cholangitis [PSC]), and genetic metabolic diseases (he-mochromatosis and Wilson disease) [see Table 1]. With the current epidemic of obesity, nonalcoholic steatohepatitis (NASH) is increasingly being recognized as a major cause of cirrhosis. Many patients diagnosed with cryptogenic cirrhosis have a history of metabolic syndrome, suggesting a role for NASH in the pathogenesis of their cirrhosis.
Many genes interact with environmental factors to cause cir-rhosis.6 Nongenetic factors that influence progression to cirrhosis include age, alcohol intake, immunosuppressive therapy, and HIV infection. Genetic factors involved in the pathogenesis of cirrhosis are not well known, but they may explain the broad spectrum of responses to the same etiologic agent found in patients with chronic liver disease. Polymorphisms in genes encoding immunoregulatory proteins, inflammatory cytokines, and fi-brogenic mediators influence the occurrence of conditions that may cause chronic liver injury (e.g., alcohol abuse, chronic HCV infection, and autoimmune disorders), as well as modulate the progression of chronic hepatitis to cirrhosis.
Pathogenesis
Early phase: liver fibrogenesis
Cirrhosis is the end stage of many forms of chronic liver disease that are characterized by progressive fibrosis. Hepatic fibro-sis is the result of the wound-healing response of the liver to repeated injury.7 It consists of the accumulation of extracellular matrix (ECM) proteins, mainly fibrillar collagen, from both increased ECM synthesis and decreased degradation. Myofibro-blasts, mostly derived from hepatic stellate cells, are the main ECM-producing cells in the injured liver. Chronic injury promotes the activation of stellate cells into fibrogenic myofibro-blasts [see Figure 1]. Key mediators of this process include inflammatory cytokines, transforming growth factor-1 (TGF-1), and angiotensin II. The pathogenesis of liver fibrosis varies with the underlying cause. In alcohol-induced liver disease, lipopoly-saccharide levels are elevated in portal blood; the lipopolysac-charide activates Kupffer cells to release reactive oxygen species and cytokines, activating stellate cells and sensitizing hepato-cytes to undergo apoptosis. The pathogenesis of HCV-induced liver fibrosis is poorly understood. HCV infects hepatocytes, causing oxidative stress and inducing the recruitment of inflammatory cells. Both factors lead to stellate cell activation. In chronic cholestatic disorders, such as PBC, T cells and cytokines mediate persistent bile duct damage.
Table 1 Main Causes of Cirrhosis
|
Viral diseases |
Hepatitis B |
|
Hepatitis C |
|
|
Hepatitis D |
|
|
Autoimmune diseases |
Autoimmune hepatitis |
|
Primary biliary cirrhosis |
|
|
Primary sclerosing cholangitis |
|
|
Graft versus host disease |
|
|
Hepatotoxic agents |
Alcohol abuse |
|
Drugs (e.g., methotrexate, a-methyldopa, amiodarone) |
|
|
Vitamin A intoxication |
|
|
Acquired metabolic diseases |
Nonalcoholic steatohepatitis |
|
Vascular diseases |
Chronic right-sided heart failure |
|
Budd-Chiari syndrome |
|
|
Veno-occlusive disease |
|
|
Inferior vena cava thrombosis |
|
|
Genetic diseases |
Wilson disease |
|
Hemochromatosis |
|
|
Type IV glycogen storage disease |
|
|
Tyrosinemia |
|
|
^-Antitrypsin deficiency |
|
|
Miscellaneous |
Secondary biliary cirrhosis Cryptogenic |
Figure 1 Immunohistochemical analysis of accumulation of fibrogenic myofibroblasts (smooth muscle [a-actin-positive] cells) in a liver biopsy specimen from a 56-year-old man with liver cirrhosis from chronic hepatitis C infection. The patient was admitted for the study of new-onset ascites. Myofibroblasts mainly accumulate in fibrous septa. Some activated hepatic stellate cells can be observed around hepatic sinusoids (arrow). (a) Magnification: x40; (b) magnification: x600.
Biliary cells secrete fibrogenic mediators that activate neighboring portal myofibroblasts to secrete ECM. Eventually, perisinusoidal stellate cells become activated and fibrotic bands develop. The pathogenesis of liver fi-brosis in NASH is poorly understood, but a so-called two-hit model has been proposed: first, hyperglycemia and insulin resistance lead to elevated serum levels of free fatty acids, resulting in hepatic steatosis; second, oxidative stress and inflammatory cy-tokines promote hepatocyte apoptosis and the recruitment of inflammatory cells, leading to progressive fibrosis.
Cirrhosis
Bridging fibrosis is associated with profound abnormalities in hepatic microcirculation.8 Capillarization of the hepatic sinusoids occurs, and new vessels form within the fibrous sheath. There is a local predominance of vasoconstrictors over vasodilators, resulting in a tonic contraction of perisinusoidal stellate cells that increases vascular resistance. Moreover, thrombosis in small vessels occurs and intrahepatic arterial shunts develop. Hepato-cytes proliferate in ischemic areas in a disorganized manner, forming regenerative nodules. Pressure in the portal venous system progressively increases, leading to the development of por-tocollateral veins and esophageal varices.9 The resulting portal hypertension leads to splanchnic vasodilatation, which increases hepatic venous blood flow. Systemic vascular resistance is decreased, and eventually, there is a marked activation of systemic vasoconstrictor systems that worsen portal hypertension and favor ascites formation. Hepatocellular function is progressively impaired, and there is decreased function of the reticuloendo-thelial system, leading to endotoxemia and the increased risk of bacterial infections. Eventually, hepatocellular function fails, resulting in severe coagulopathy and hepatic encephalopathy. A profound circulatory dysfunction from impaired myocardial function and decreased systemic vascular resistance is frequently seen. In very late stages of cirrhosis, renal vasoconstriction develops, leading to the hepatorenal syndrome (HRS). In this phase of the disease, most patients die unless an OLT is rapidly performed.
Diagnosis
The diagnostic process in a patient with suspected cirrhosis is intended to determine the presence, severity, and cause of the condition. Data obtained from the history, physical examination, laboratory tests, and liver biopsy are used to identify the etiology of cirrhosis [see Table 2].
Clinical manifestations
Cirrhosis can be clinically silent, and some cases are discovered incidentally at laparotomy or autopsy. In many patients, symptoms are insidious in onset and include generalized weakness, anorexia, malaise, and weight loss. Skeletal muscle mass is frequently reduced. So-called compensated cirrhosis is defined by the absence of symptoms or the presence of only minor symptoms. Eventually, most patients exhibit the clinical manifestations of hepatocellular dysfunction and portal hypertension, including progressive jaundice, bleeding from gastroesophageal varices, ascites, and neuropsychiatric symptoms. The abrupt onset of one of these complications may be the first manifestation of cirrhosis. Coagulopathy and subsequent mucosal bleeding typically occur in patients with advanced cirrhosis. Progressive obstruction to bile flow, which is especially common in patients with PBC and PSC, leads to skin hyperpigmentation, jaundice, pruritus, and xanthelasmas. Patients who have progressed to such conditions often experience malnutrition secondary to anorexia, fat malabsorption, and increased catabolism. Deficiency of fat-soluble vitamins is also frequently found in patients with cirrhosis. In patients with alcohol-induced liver disease, extrahe-patic symptoms related to the nervous system, the heart, and the pancreas can also be present.
Physical examination findings
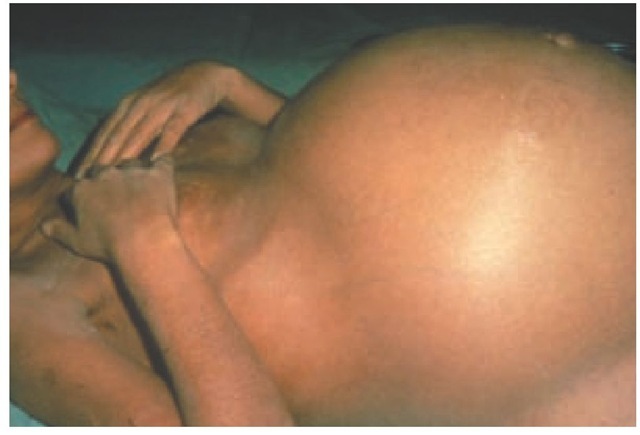
Physical examination can be normal in patients with early cirrhosis. More commonly, the liver is enlarged initially and is palpable. In advanced cirrhosis, liver size usually decreases. Splenomegaly is a common finding. Ascites, peripheral edema, or both may be present, and collateral venous circulation can be observed in the abdomen. Patients with hepatic encephalopathy have altered mental status, decreased consciousness, and asterixis. Other signs typical of cirrhosis include muscle wasting, palmar erythema, vascular spiders, gynecomastia, axillary hair loss, testicular atrophy, and fetor hepaticus. In alcoholic patients, Dupuytren contractures, parotid gland enlargement, and peripheral neuropathy can be noted. Skin hyperpigmentation is typical of patients with cholestatic disorders (e.g., PBC) or he-mochromatosis. Advanced cirrhosis is commonly marked by severe malnourishment, prominent ascites, and neuropsychiatric symptoms [see Figure 2].
Laboratory studies
Blood Tests
Liver function test results are commonly abnormal in patients with cirrhosis. Serum aspartate aminotransferase (AST) levels are frequently elevated, but levels above 300 U/L are uncommon. Serum levels of alanine aminotransferase (ALT) may be relatively low (AST/ALT ratio greater than 2). Serum prothrom-bin time is frequently prolonged, reflecting reduced synthesis of clotting proteins, most notably the vitamin K-dependent factors. Serum albumin levels are decreased, mainly because of poor he-patocellular synthesis. Total serum globulin concentration increases in advanced cirrhosis, as a result of poor reticuloen-dothelial function and increased blood levels of bacterial products. The alkaline phosphatase concentration is usually only moderately increased, except in patients with biliary diseases (i.e., PBC or PSC), who show markedly increased levels of alka-line phosphatase and y-glutamyl transpeptidase, which in some cases are associated with increased bilirubin levels.
Table 2 Identification of the Main Causes of Cirrhosis
|
Cause |
Diagnostic Method |
|
Alcohol-related |
Medical history (also obtained from relatives), urinary alcohol levels, histologic findings |
|
Hepatitis C virus (HCV) |
Anti-HCV antibodies, HCV RNA assay |
|
Hepatitis B virus (HBV) |
HBsAg, HBV DNA assay |
|
Hepatitis D virus (HDV) |
Anti-delta IgM or IgG |
|
Autoimmune disease |
Antitissue antibodies (ANA, LKM, ASMA), hypergammaglobulinemia, histologic findings |
|
Primary biliary cirrhosis |
AMA, histologic findings |
|
Primary sclerosing cholangitis |
Severe cholestasis; detection of biliary tract abnormalities by magnetic resonance imaging/magnetic resonance cholan-giopancreatography (MRI/MRCP) or ERCP; ANCA; presence of inflammatory bowel disease; histologic findings |
|
Wilson disease |
Serum ceruloplasmin levels, Kayser-Fleischer rings, copper content in the liver, genetic studies |
|
Hemochromatosis |
Serum ferritin levels, total iron binding capacity, iron content in the liver, genetic studies |
|
Nonalcoholic steatohepatitis |
Metabolic syndrome, histologic findings (may be absent in cirrhosis), absence of alcohol abuse |
Figure 2 Photograph of a 45-year-old patient with advanced cirrhosis from alcohol-induced liver disease. The patient was admitted because of tense ascites, and a superimposed acute alcoholic hepatitis was diagnosed. A large-volume paracentesis followed by albumin administration was performed.
Anemia is fairly common; it is usually normocytic, but it may be microcytic, hypochromic from chronic GI bleeding, macrocytic from folate deficiency (in alcoholism), or hemolytic. Hypersplenism can lead to leukopenia and thrombocytopenia. Cholesterol and triglyc-eride levels may be increased in patients with biliary obstruction, whereas they are low in patients with advanced cirrhosis of non-biliary origin. Cirrhotic patients may develop glucose intolerance and diabetes mellitus, mainly because of insulin resistance. Central hyperventilation may lead to respiratory alkalosis. Dietary deficiency and increased urinary losses cause hypomagne-semia and hypophosphatemia. Renal failure, as indicated by elevated creatinine and blood urea nitrogen levels, and hypona-tremia can be observed in cirrhotic patients with ascites.
Imaging Studies
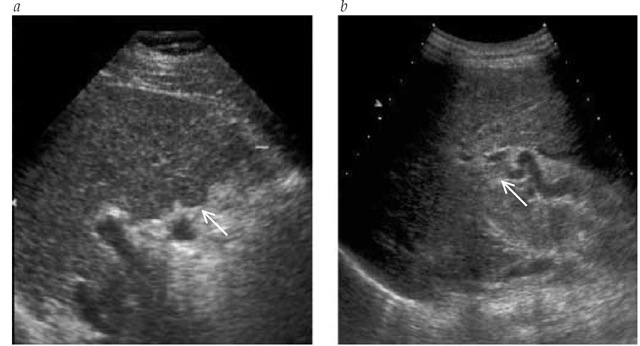
Real-time ultrasonography, in combination with color flow Doppler, is the most useful tool in the evaluation of patients with cirrhosis.10 Ultrasonography is useful for demonstrating the morphologic characteristics of cirrhosis, including irregular or nodular liver edges, altered structure, and signs of portal hypertension such as portocollateral veins. It is also useful to detect hepatic steatosis, ascites, splenomegaly, and portal vein thrombosis. In patients with cholestasis, ultrasonography helps rule out extra-hepatic causes of jaundice. Doppler ultrasonography provides useful information on portal hemodynamics and can detect reversal of portal blood flow [see Figure 3]. Ultrasound examination is particularly helpful for detecting hepatic tumors such as HCC. Demonstration of tumor vascularization by Doppler ultrasonog-raphy, with or without injection of ultrasound contrast, is valuable in the differentiation of regenerating nodules from HCC. Dynamic studies using computed tomography and magnetic resonance imaging are also useful in the assessment of cirrhosis and the diagnosis of hepatic tumors previously detected by ultrasonography. The use of CT or MRI to screen for HCC in patients with cirrhosis is limited by the high cost of these techniques.
Liver Biopsy
Liver biopsy can unequivocally establish the presence of cirrhosis.
Figure 3 Real-time ultrasound images of a 56-year-old man with liver cirrhosis from chronic hepatitis C. The patient had a compensated cirrhosis and was undergoing liver ultrasonography plus determination of a-fetoprotein serum levels every 6 months to screen for hepatocellular carcinoma. (a) The liver showed irregular edges (arrow) and an altered structure. (b) A patent portal vein thrombosis was detected (arrow).
Liver biopsy helps determine the cause of cirrhosis, as well as provides information on the extent of liver damage. The biopsy is usually performed using a percutaneous approach, but percutaneous biopsy should not be used in patients with severe coagulopathy (i.e., those with an international normalized ratio [INR] greater than 1.5 or a platelet count less than 50,000/^l), and it must be used with caution in patients with ascites or severe obesity. Limitations of liver biopsy are that it is an invasive procedure and that sampling error can occur (i.e., false negative results), especially in patients with macronodular cirrhosis.
Transjugular liver biopsy offers an alternative to percutaneous biopsy. Transjugular liver biopsy can be used in patients with ascites; is indicated in patients with severe coagulopathy; and allows the measurement of portal pressure.12 However, the amount of tissue obtained is limited, and often, the diagnosis of cirrhosis cannot be made. In selected cases, liver biopsy can be performed during laparoscopy. This approach is generally reserved for the staging of cancer or for ascites of unknown origin.
Histologic findings that define cirrhosis include extensive fi-brosis and regenerative nodules. The degree of infiltration of inflammatory cells depends on the activity of the underlying disease. Micronodular cirrhosis is characterized by the presence of uniformly small nodules (diameter < 3 mm), whereas in macro-nodular cirrhosis, nodules vary in size (diameter 3 mm to 5 cm) and contain some normal lobular structure (e.g., portal tracts or terminal hepatic venules).
In some cases, histologic findings help identify the causative agent of cirrhosis, such as periportal lymphocyte infiltration in HCV-induced cirrhosis; Mallory bodies, polymorphonuclear leukocyte (PMN) infiltration, and steatosis in alcohol-induced cirrhosis and NASH; biliary involvement in PBC; and massive iron deposition in hemochromatosis. In advanced cirrhosis, however, different underlying diseases may have similar histologic findings.
![Immunohistochemical analysis of accumulation of fibrogenic myofibroblasts (smooth muscle [a-actin-positive] cells) in a liver biopsy specimen from a 56-year-old man with liver cirrhosis from chronic hepatitis C infection. The patient was admitted for the study of new-onset ascites. Myofibroblasts mainly accumulate in fibrous septa. Some activated hepatic stellate cells can be observed around hepatic sinusoids (arrow). (a) Magnification: x40; (b) magnification: x600. Immunohistochemical analysis of accumulation of fibrogenic myofibroblasts (smooth muscle [a-actin-positive] cells) in a liver biopsy specimen from a 56-year-old man with liver cirrhosis from chronic hepatitis C infection. The patient was admitted for the study of new-onset ascites. Myofibroblasts mainly accumulate in fibrous septa. Some activated hepatic stellate cells can be observed around hepatic sinusoids (arrow). (a) Magnification: x40; (b) magnification: x600.](http://what-when-how.com/wp-content/uploads/2012/04/tmp4461_thumb_thumb.jpg)