Diagnosis
Three types of bronchiectasis have been described on the basis of bronchographic-pathologic findings: cylindrical, varicose, and cystic. Distinction between these three types of bronchiecta-sis is not useful clinically, however, because the manifestations and course of bronchiectasis are not correlated with the bron-chographic pattern.
Clinical manifestations In most cases, the clinical presentation and a plain chest radiograph suffice for a presumptive diag nosis of bronchiectasis. Factors in the history, such as chronic cough and sputum purulence originating from a serious respiratory tract infection, often in childhood, strongly suggest the diagnosis. In addition, chronic sinusitis frequently accompanies bronchiectasis, and its presence should raise the suspicion of concomitant chronic lower respiratory tract infection.
In other patients, the clinical picture is one of frequent lower respiratory tract infections limited to the same area or areas of the lungs. Symptoms, physical findings, and abnormalities on the chest radiograph may not clear completely between each episode of pneumonia. Some patients have a mucoid sputum that becomes intermittently infected; disease course mimics that of chronic bronchitis with episodic infectious exacerbations. In this case, the local findings on chest examination and on the chest radiograph usually point to the diagnosis of bronchiectasis.
Clubbing of the digits occurs in the majority of patients with significant bronchiectasis and is a valuable diagnostic clue, especially because clubbing of the digits is not a manifestation of COPD [see Chronic Bronchitis and Emphysema, Diagnosis, above]. Auscultation of the chest usually reveals localized findings. Typically, paninspiratory, coarse crackles are heard over the involved region, and there may also be variable low-pitched wheezes if secretions are present in the airways.
Chest imaging Bronchiectasis may be seen on the plain chest radiograph in a number of different patterns. Cystic bronchiectasis is most readily recognized because of the distinctive appearance of a collection of thin-walled cystic spaces, sometimes accompanied by air-fluid levels, arranged in a seg-mental distribution. A localized increase in interstitial markings that follow the general orientation of the bronchovascular bundles may indicate milder degrees of ectasia. Occasionally, the thickened walls of a dilated bronchus can be visualized as the bronchus courses with its longitudinal axis perpendicular to the x-ray beam. These parallel lines are approximately 1 mm thick and are referred to as tramlines. Atelectasis often accompanies extensive bronchiectasis, in which case the radiographic appearance may mimic a postobstructive pneumonia. In approximately 7% of patients with bronchiectasis, the plain chest radiograph is entirely normal.
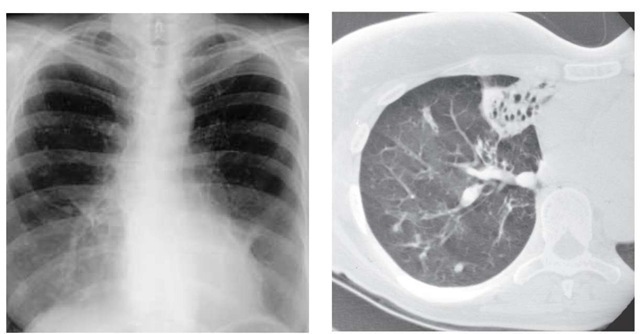
The current generation of CT machines provide excellent magnified images of bronchiectatic airways. CT scanning can be used to confirm a clinical suspicion of bronchiectasis, to suggest the specific cause,124 and to evaluate the extent of disease125 [see Figure #].
Sputum examination Examination of purulent sputum produced by a patient with bronchiectasis may suggest the underlying diagnosis in two ways. First, if sputum is collected in a container for several hours until a sufficient volume is obtained, the sputum may settle into a characteristic three-layered pattern: foamy on top, purulent in the middle, and liquid at the bottom. Occasionally, the same pattern is observed in sputum from patients with chronic bronchitis or suppurative lung abscess. Second, routine bacterial culture of the sputum may grow Pseudo-monas species. In an immunocompetent host, Pseudomonas species are almost never isolated from the sputum unless the host has bronchiectasis, is receiving broad-spectrum antibiotics, has a long-term tracheostomy, or is in the intensive care unit. Staphylo-coccus aureus, gram-negative bacilli other than Pseudomonas (especially H. influenzae), and Mycobacterium avium complex126 may also infect the airways of patients with bronchiectasis. Bronchiec-tasis is also a feature of allergic bronchopulmonary aspergillosis [see 14:II Asthma].
Figure 8 In a comparison of a frontal chest radiograph with a CT scan of a patient with hemoptysis, the radiograph (left) suggests only an infiltrate in the right middle lobe; the CT scan (right) shows bronchiectasis in the right middle lobe that was the source of the bleeding.
Pulmonary function tests Pulmonary function tests may remain normal if only a small portion of the tracheobronchial tree is affected. Widespread bronchiectasis causes chronic obstruction of expiratory airflow and may also cause a restrictive deficit if there is sufficient associated atelectasis or involvement of lung parenchyma by the infectious process. However, airflow obstruction is generally the main abnormality.
Treatment
The mainstays of therapy for bronchiectasis (including cystic fibrosis and primary ciliary dyskinesia), as for any chronic sup-purative disease, are administration of antibiotics and drainage.
Antibiotics The use of antibiotics in the treatment of bron-chiectasis has not been subjected to careful scientific investigation, and no one method of administration has proved to be superior in clinical experience. It is reasonable to culture the sputum periodically, because in patients from whom S. aureus or H. influenzae has repeatedly been isolated, antibiotics with appropriate spectra of activity can be selected. Oral antibiotics with effective antipseudomonal activity, such as the quinolones, show promise; however, because of the potential emergence of resistant strains of Pseudomonas, these drugs probably should not be used as single agents for long-term suppressive therapy. Nevertheless, many patients seem to benefit from broad-spectrum oral antibiotics, even when Pseudomonas is the only pathogen in the sputum and in vitro sensitivity testing shows that the antibiotics lack activity against Pseudomonas. In a patient who has daily purulent sputum production and is not allergic to sulfon-amides, trimethoprim-sulfamethoxazole (one double-strength tablet twice daily) can be given continuously. Alternatively, antibiotics may be given intermittently or on a schedule in which different antibiotics are rotated.127 Nebulized antibiotics may also be effective.128
If oral antibiotic therapy has failed, serious infectious complications (such as persistent fever with new areas of infiltration detected on the chest radiograph or the development of pleuritic chest pain) are generally best treated with a 10- to 14-day course of intravenous antibiotics. Two synergistic antibiotics with appropriate in vitro activity should be used for Pseudomo-nas infection.
Drainage Drainage of secretions is partially achieved by coughing and expectoration of sputum. However, because the diseased airways collapse during coughing and forced exhalation and because there is pooling of secretions distal to the areas of collapse, it is useful to include postural drainage as part of the management of bronchiectasis. Chest physiotherapy (e.g., the use of chest percussion and vibration) is often used to aid bron-chopulmonary drainage, although it is difficult to demonstrate that physiotherapy produces benefits beyond those that are produced by postural drainage alone. There is no role for aerosolized recombinant human deoxyribonuclease (DNase) in bron-chiectasis not associated with cystic fibrosis.
Bronchodilator therapy Many patients with bronchiectasis will have significant airflow obstruction, manifested clinically by wheezing and detected by pulmonary function testing. Theo-phylline, beta2 agonists, and anticholinergic bronchodilators can be used in this setting, although the evidence for their effectiveness is limited.130-133 Bronchodilator therapy may promote the clearance of airway secretions if the bronchodilating agents are administered before each postural drainage session.
Anti-inflammatory therapy During episodes of exacerbation, oral corticosteroids may help improve the patient’s condition, although the benefit of this approach has not been established in randomized trials.134 High-dose inhaled corticosteroids have been shown to reduce markers of airway inflammation135 and improve lung function,136 but the potential role of long-term therapy is uncertain. Leukotriene modifiers have no role in the treatment of bronchiectasis, except in the setting of allergic bron-chopulmonary aspergillosis.
Therapy for hemoptysis Significant hemoptysis can also usually be controlled with appropriate antibiotic therapy. Massive hemoptysis (> 200 ml of blood over a 24-hour period) that occurs as a complication of bronchiectasis was traditionally managed with surgical resection of the involved lung. Now, however, massive hemoptysis caused by bronchiectasis is often effectively treated with bronchial arterial embolization, an invasive radiologic procedure involving catheterization of the bronchial arteries. The dilated bronchial arteries that perfuse the airways in bronchiectasis are particularly suited for the application of this technique. The procedure requires a skilled angiographer.
Surgery In the modern antibiotic era, the role of surgery in the management of bronchiectasis has been declining.138 In patients with widespread bilateral disease, diseased lung tissue is better able to support gas exchange than no lung tissue at all. In patients with only limited localized disease, symptoms can usually be well controlled with the measures described above. In addition, experience indicates that after lobar resections for localized bronchiectasis have been performed, clinically evident recurrences of the disease are common in parts of the lung that had previously been thought to be uninvolved. In the rare instance in which severe symptoms or recurrent complications in a young patient lead to consideration of resection, the localized nature of the bronchiectasis must first be demonstrated radi-ographically. If there is bronchiectasis distal to an obstructing bronchial lesion, surgery is indicated to remove the obstruction along with the diseased lung tissue.
Clinical variants of bronchiectasis
Cystic Fibrosis
Although cystic fibrosis is an inherited disease that usually manifests itself in early childhood, a discussion of the condition in the context of general adult medicine is worthwhile for two reasons. First, increasing numbers of children with cystic fibrosis are now surviving into young adulthood: the median survival in the United States is 32 years.139 Second, some patients have a variant form of the disease in which symptoms first appear during adolescence or adulthood.
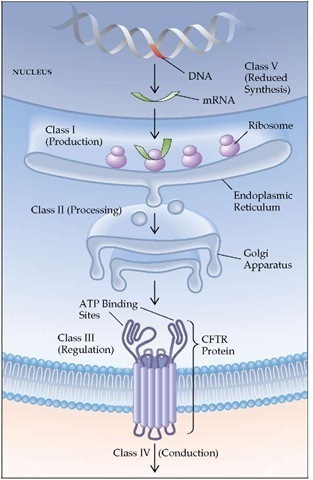
Pathogenesis The genetic defects responsible for cystic fi-brosis have been identified.139 The cystic fibrosis locus is on the long arm of chromosome 7; it codes a polypeptide comprising 1,480 amino acids that has been named the cystic fibrosis transmembrane regulator (CFTR).139 In 70% of patients with cystic fibrosis, the 508th amino acid of this sequence is missing (AF508 mutation). The abnormal protein derived from the altered sequence is not glycosylated; it is retained in the endoplasmic reticulum and is not transferred to the cell membrane. The result is a defective membrane with decreased apical chloride conductance and increased sodium absorption.140 Excessive dehydration of respiratory secretions may alter the character of the sol phase, in which the cilia normally beat, making it thicker and more viscous. Patients who are homozygous for the AF508 mutation have a more severe form of the disease than those who are heterozygous.141 A number of other defects of the cystic fibrosis gene have also been identified, and the resultant defects in the production of CFTR can be grouped into five classes [see Figure 9]. Because these defects cause the CFTR to function differently, phenotypic severity differs among the classes.142 Several of these defects are associated with mild lung disease and even normal sweat chloride concentrations.143 As a result, patients may present at a later age, and diagnosis may be difficult. In addition, polymorphisms of other genes involved in the immune response may alter the phenotypic severity.
It is likely that impaired tracheobronchial clearance of the abnormal secretions leads to widespread mucous plugging of airways, resulting in secondary bacterial infection, persistent inflammation, and consequent generalized bronchiectasis.145 The bacterial flora in the airways are highly stereotyped: early in the course of the disease, S. aureus is found in the sputum; subsequently, mucoid strains of P. aeruginosa are isolated (mucoid in this context refers to a slimy substance secreted by the colony of organisms growing on a culture plate). Despite the presence of these highly virulent pathogens in the lower respiratory tract, infection remains confined to the airways. Although lung abscess and empyema are common complications of Staphylococ-cus or Pseudomonas pneumonia, they very rarely develop in patients with cystic fibrosis.
Diagnosis CAO is present in virtually all adult patients with cystic fibrosis and follows a relentlessly progressive course. Thus, cough, chronic purulent sputum production, and exer-tional dyspnea are cardinal symptoms of cystic fibrosis. Increased airway reactivity is found in approximately 20% to 25% of patients with this disease; in this subgroup, episodic wheezing may be a prominent manifestation, leading to a misdiagno-sis of asthma.146 Nasal polyposis and chronic sinusitis are common upper respiratory tract findings in patients with cystic fi-brosis and may be mistaken for signs of allergic disease. Even findings consistent with allergic bronchopulmonary aspergillo-sis are observed in as many as 9% of patients.145 A small number of patients become colonized, although they are rarely infected, with atypical mycobacteria.145,147 Two important complications of lower respiratory tract disease in patients with cystic fibrosis are hemoptysis and pneumothorax. Minor hemoptysis occurs intermittently in a majority of patients. In approximately 7% of adult patients, rupture of dilated bronchial arteries leads to massive, potentially fatal hemoptysis. Pneumothoraces may complicate the course of advanced obstructive lung disease in approximately one sixth of adult patients and are frequently recurrent.148 Hy-poxemia and hypercapnia, occurring initially during exercise and sleep,149 are prominent complications and can be associated with the development of pulmonary hypertension.150 Osteoporosis producing significant kyphosis is frequent in adult patients with cystic fibrosis.151
The chest radiograph may strongly suggest the diagnosis of cystic fibrosis. The generalized bronchiectasis manifests itself as a diffuse increase in interstitial markings, and discrete bron-chiectatic cysts are often visible; typically, involvement of the upper lobes predominates. The obstructive aspect of the disease is reflected in the typical finding of pulmonary hyperinflation. This combination of diffusely increased markings with cystic spaces, upper lobe predominance, and hyperinflation is highly characteristic of cystic fibrosis; rare alternative radiographic diagnoses include eosinophilic granuloma and lymphangiomy-omatosis [see 14:V Chronic Diffuse Infiltrative Lung Diseases]. In the late stages of the disease, cardiomegaly and signs of pulmonary arterial hypertension appear on the chest radiograph as cor pul-monale develops.
Extrapulmonary manifestations may also suggest the diagnosis of cystic fibrosis. Prominent among these findings are pancreatic insufficiency with consequent steatorrhea, recurrent partial intestinal obstruction caused by abnormal fecal accumulation (so-called meconium ileus equivalent), heat prostration, hepatic cirrhosis, and aspermia in males.
The diagnosis of cystic fibrosis should therefore be suspected in the adolescent or young adult who has widespread bronchi-ectasis and Staphylococcus or Pseudomonas infection of the airways. The diagnosis should also be considered in the young patient with so-called refractory asthma, especially if the asthma symptoms are accompanied by clubbing of the digits, chronic sputum purulence, a persistently abnormal chest radiograph, or symptoms of pancreatic insufficiency. The diagnosis can be established by a finding of abnormal results on a sweat test performed in a qualified laboratory using pilocarpine iontophore-sis.152 In persons younger than 20 years, a sweat chloride level exceeding 60 mEq/L confirms the diagnosis; a value exceeding 80 mEq/L is required for diagnosis in persons 20 years of age or older. The diagnosis can be confirmed by genotyping for the most common CFTR mutations. Genotyping should also be used when the sweat chloride tests results are equivocal or normal and cystic fibrosis is strongly suspected.
With the identification of the gene for cystic fibrosis, genetic screening has become available. A National Institutes of Health panel suggested that genetic testing for cystic fibrosis be offered to adults with a positive family history of the disease, to partners of people with the disease, to couples currently planning a pregnancy, and to couples seeking prenatal care, but not to the general population or newborns.
Treatment Treatment of the pulmonary aspects of cystic fibrosis is similar to that of bronchiectasis [see Bronchiectasis, Treatment, above] and includes management of infections and the use of respiratory therapy modalities that are designed to mobilize secretions, including regular percussion and postural drainage, and to reduce airway obstruction.139,145,154 A pneumatic bronchial drainage vest or a flutter device makes it easier to vibrate the chest so as to enhance the removal of thick secre-tions.155,156 No randomized, controlled trials have established the efficacy of any airway clearance regimen in cystic fibrosis, how-ever.157 Aerosolized antibiotics such as tobramycin may have a role in reducing the burden of infection in those who have become chronically infected with P. aeruginosa.115,158 Treatment with intravenous antibiotics is usually required for episodes of symptomatic infection with P. aeruginosa145,159 or other organisms. Chronic treatment with macrolide antibiotics may have beneficial effects related to mechanisms other than antibacterial activi-ty—namely, reduction in biofilm formation and anti-inflammatory properties.160 Because the viscosity of the mucus in cystic fi-brosis is partially caused by DNA released from cells, re-combinant human DNase administered by inhalation is effec-tive.161 In patients with reversible airflow obstruction, treatment with bronchodilators (e.g., beta agonists), anticholinergics, and theophylline and with low-dose alternate-day oral or daily inhaled corticosteroids may be of benefit.162-164 Strategies to reduce airway inflammation and to reduce the burden of neutrophil proteases are also being evaluated.165,166 Attention to nutrition, physical conditioning, and emotional health must be part of an effective care plan. Bronchial artery embolization is useful in patients with significant hemoptysis.167 Mechanical ventilatory support at night using noninvasive techniques may be useful in patients with chronic respiratory failure.168 Lung transplantation is now being performed with good results in cystic fibrosis patients whose FEV1 is less than 30% of predicted value.
Figure 9 Categories of cystic fibrosis transmembrane conductance regulator (CFTR) mutations. Cystic fibrosis can be produced by abnormalities at several points in the pathway from gene to functional protein on the cell surface. Class I mutants are associated with decreased transcription of the DNA or translation of the RNA. Class II mutants are attributable to abnormalities in processing of the protein in the endoplasmic reticulum, resulting in the degradation of the protein. Class III mutants are associated with abnormal regulation of the protein. Class IV mutants are associated with abnormal function of the CFTR protein on the cell surface. Class V mutants are associated with decreased synthesis of the CFTR protein. Some mutants (e.g., AF508, class II and class III) can be associated with more than one defect.
The discovery of a specific genetic defect raises the possibility of more specific and perhaps more effective therapy. Therapy could be either pharmacologic (aimed at altering transport through involved or uninvolved ion channels) or genetic (aimed at replenishing the CFTR). In pilot studies, inhalation of amiloride, an epithelial sodium channel blocker, led to objective improvement in sputum character. Other sodium channel blockers that are more potent and longer-acting may be available in the future. Triphosphate nucleotides (adenosine triphos-phate and uridine triphosphate) have been found to be effective chloride secretagogues in vivo but have not been tested in long-term therapy.171 Another approach is to rescue the mutant CFTR protein from the endoplasmic reticulum and improve the function of the abnormal channel when it is delivered to the plasma membrane.172 Strategies aimed at correcting the genetic defect are advancing, albeit slowly.108 An altered adenovirus (incapable of replication) has been used to introduce the cystic fibrosis gene into patients, and phase II trials of this therapy are under way.108 Several other approaches are being taken and may be successful in the near future.
Primary Ciliary Dyskinesia
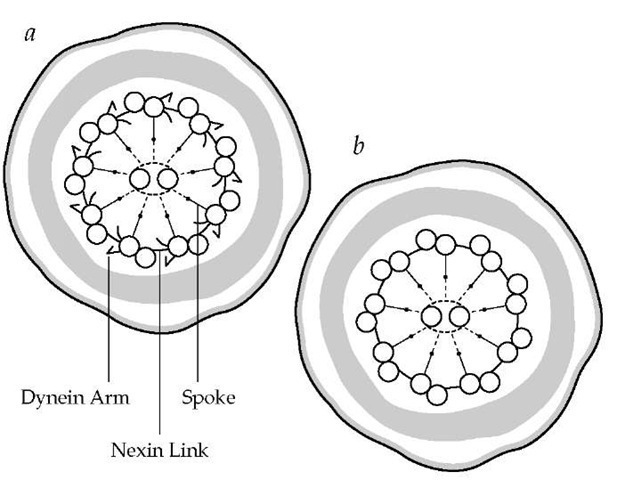
In 1933, Dr. Manes Kartagener identified a group of patients with bronchiectasis who also suffered from chronic sinusitis and situs inversus. This triad of findings came to be known as Karta-gener syndrome. Approximately 40 years later, it was recognized that male infertility was associated with this syndrome: men with Kartagener syndrome were found to have live sperm with absent or ineffective motility. Sperm tails and the cilia of respiratory tract epithelial cells share an ultrastructure, and in 1975, it was recognized that an inherited abnormality in that ul-trastructure (i.e., an absence of the adenosine triphosphatase [ATPase]-containing dynein arms of the outer microtubular doublets) led to nonfunctioning respiratory tract cilia and im-motile spermatozoa174 [see Figure 10].
The consequences of congenital nonfunctioning cilia of the upper and lower respiratory tracts are chronic sinusitis, secretory otitis media, and daily productive cough dating from birth or early childhood; bronchiectasis develops during childhood in the majority of patients. With respect to situs inversus, it is speculated that the normal asymmetrical positioning of body organs is dependent on normal functioning of cilia on embryonic epithelium. In the absence of normal ciliary function, placement of organs to either the left or the right is random, and as expected, about one half of patients with congenitally nonfunctioning cilia manifest situs inversus. Thus, the term immotile cilia syndrome was coined to include all patients with chronic sinusitis and bronchiectasis resulting from ultrastructural abnormalities of cilia. Fertility is reduced not only in men but also in women with this syndrome, because of deficient cilia in the oviducts and fimbriae.
Primary ciliary dyskinesia highlights the importance of normal ciliary function in clearing airway mucus, as well as the importance of other mechanisms, especially cough, in defending against disease. Ciliary dysfunction in the lower respiratory tract leads to the retention of secretions, bacterial superinfection, and bronchiectasis. Other protective mechanisms appear to prevent more serious sequelae, including acute pneumonias and progressive airflow obstruction.
Pathogenesis A variety of abnormalities in addition to absent or deficient dynein arms may impair the structure and function of cilia and sperm tails.175 Many of these abnormalities involve derangement of the normal configuration of micro-tubules. In each case, in vitro microscopic studies of ciliary motility have demonstrated a pattern of decreased, uncoordinated, or ineffective beating. Because at least some movement of the cilia is observed, the term primary ciliary dyskinesia has been proposed as a more accurate description of this condition than the term immotile cilia syndrome. Inheritance is thought to be autosomal recessive. Any given ultrastructural abnormality is found consistently in the cilia of the upper and lower respiratory tract (as well as in sperm tails in men), and all affected members of the same family have the same defect. The clinical syndrome that results is a common expression of impaired ciliary motility independent of the specific ultrastructural defect.
Figure 10 A cross section (a) of the tail of a normal sperm reveals dynein arms. In a cross section of the tail of a sperm from a patient with Kartagener syndrome (b), dynein arms are missing; such a cell would be immotile. Similar changes have been noted in the cilia in the respiratory tract and paranasal sinuses in these patients.
Diagnosis The diagnosis of primary ciliary dyskinesia can be made clinically in all patients with Kartagener syndrome.176 A clinical diagnosis can also be made on the basis of the following criteria: a history of chronic sinusitis; a productive cough since childhood; and either the presence of live but immotile spermatozoa or a family history in which the patient has a sibling with Kartagener syndrome. In patients who have had chronic sinusitis and productive cough since childhood but who meet neither of the two additional criteria, three types of specialized laboratory studies can be employed to support the diagnosis of primary ciliary dyskinesia: (1) electron microscopic examination of sperm tails or of cilia from bronchial or nasal biopsy specimens, (2) in vitro light microscopic examination of the motility of cilia, and (3) measurement of mucociliary clearance in the nose or the tracheobronchial tree. An example of the last technique listed is inhalation of an aerosol of radiolabeled particles followed by external scanning over the thorax for at least 2 hours. Normal values for the ciliary structural and functional studies have been established.177 These studies should be performed during periods of clinical stability, because acute inflammation can reversibly alter ciliary function.178
Differential diagnosis The respiratory disease associated with primary ciliary dyskinesia may be contrasted with that associated with cystic fibrosis. In cystic fibrosis, in the absence of acute infectious exacerbations, ciliary function is normal and mucociliary transport only modestly decreased. In primary ciliary dyskinesia, however, mucociliary transport along nasal or tracheobronchial mucosa is virtually absent. Nevertheless, disease of the lower respiratory tract is usually far milder in patients with primary ciliary dyskinesia than in those with cystic fibrosis. Bronchiectasis in primary ciliary dyskinesia usually involves the lower or the middle lung zones and is less widespread than in cystic fibrosis. Also, in primary ciliary dyskinesia,bacterial infection of the airways is more commonly caused by Haemophilus, Neisseria, or Streptococcus organisms than by Staphylococcus or Pseudomonas organisms, and acute pneumonias are relatively infrequent. Finally, airflow obstruction is usually mild in primary ciliary dyskinesia, and progression to cor pulmonale is uncommon. Patients with primary ciliary dyskine-sia usually can remain fully active and may survive to old age.
Treatment Treatment with postural drainage and antibiotics for infections aids in maintaining stable lung function [see Bronchiectasis, Treatment, above].
Bronchiolitis
Disorders of the bronchioles can be divided into three categories: (1) primary bronchiolar disorders, which are covered in this section; (2) parenchymal disorders with prominent bronchi-olar involvement, and (3) bronchiolar involvement in large airway diseases.180 Parenchymal disorders with prominent bron-chiolar involvement such as hypersensitivy pneumonitis, respiratory bronchiolitis/interstitial lung disease, and bronchiolitis obliterans organizing pneumonia are covered in another topic [see 14:V Chronic Diffuse Infiltrative Lung Disease]. The larger airway diseases associated with bronchiolar involvement are COPD, bronchiectasis [see Bronchiectasis, above], and asthma [see 14:11 Asthma].
The primary bronchiolar disorders can be further divided into five types: acute bronchiolitis, constrictive bronchiolitis (obliterative bronchiolitis or bronchiolitis obliterans), diffuse panbronchiolitis, mineral dust airway disease, and follicular bronchiolitis.
Etiology
Acute bronchiolitis is mostly a disease of childhood.180 One form of the disease, an acute bronchiolitis that occurs in infants, is often the result of infection with respiratory syncytial virus. Adenovirus infection may cause a more serious necrotizing form of bronchiolitis in children; in some of these children, the healing process is characterized by exuberant inflammation and fibrosis that obliterate the bronchiolar lumen. If only one lung is affected, the obstructive disease may appear on radiography as a unilateral hyperlucency of the lung field because of distal alveolar overdistention (i.e., air trapping) and decreased vascularity in the affected lung. This syndrome of a unilateral hyperlucent lung in a patient with bronchiolitis bears two eponyms: Swyer-James syndrome, named for the two physicians who first described the disease in children, and Macleod syndrome, named after the physician who reported the first adult case.
Constrictive bronchiolitis is a rare cause of CAO in adults, occurring after viral pneumonia (e.g., pneumonia caused by measles, influenza, or adenovirus infection), after inhalation of toxic gases (e.g., chlorine or nitrogen dioxide), and as an idiopathic phenomenon.180 Constrictive bronchiolitis has been documented as a complication of collagen vascular diseases, particularly rheumatoid arthritis, and as a sequela of stem cell transplantation in the setting of graft versus host disease. A possible association with the drug penicillamine has also been suggested.180
Diffuse panbronchiolitis has been seen primarily in Asia, particularly in Japanese adults. The cause is unknown, although a genetic predisposition is suspected.
Mineral dust airway disease is associated with exposure to a number of inorganic dusts, including asbestos, iron and aluminum oxide, talc, mica, silica, silicate, and coal.
Follicular bronchiolitis can be idiopathic; it can also be associated with collagen vascular diseases (particularly rheumatoid arthritis) or immunodeficiency syndromes such as HIV infection.
Diagnosis
Clinical manifestations In patients with an acute infection or who have had a toxic exposure, fever, nonproductive cough, and dyspnea may develop 2 to 4 weeks after the initial event, often after an asymptomatic interval. In other patients, the gradual onset of dyspnea and dry cough are the presenting symptoms. Chest examination typically reveals high-pitched inspiratory crackles, wheezing, and a highly characteristic midinspiratory squeak.
Pulmonary function testing shows marked airflow obstruction and little or no reversibility of obstruction in response to bronchodilators.
Chest radiograph The characteristic chest radiographic finding is a pattern of diffuse, nodular densities, sometimes with a fine nodularity mimicking miliary tuberculosis. Although these findings are typical of bronchiolitis, a spectrum of presentations is possible. Radiographically, hyperinflation and vascular attenuation mimicking emphysema may be the only findings, or there may be scattered nonhomogeneous patchy infiltrates. High-resolution CT scanning of the chest, particularly if performed on inspiration and expiration, may show nodular lesions, regions of ground-glass attenuation, bronchocentric infiltrates, and small regions of lucency, denoting obstruction and air trapping at a small airway level.181
Treatment
Treatment of constrictive bronchiolitis is usually ineffective, although corticosteroids are often tried. Initially, high-dose corticosteroids (e.g., prednisone given in an oral dosage of 1 mg/kg/day) are used in an attempt to suppress the inflammatory reaction within and around the bronchioles. Although cor-ticosteroids often benefit patients with idiopathic bronchiolitis obliterans organizing pneumonia, they are rarely effective against other forms of bronchiolitis.
Prognosis
In some patients with bronchiolitis, the disease progresses to severe airflow obstruction, respiratory failure, and death. In others, the inflammation remits, and chest x-ray and pulmonary function test results return to normal.